Magic Spheres/Capsules
Mo132 = {Pentagon}12{Linker}30 = [{(Mo)Mo5O21(H2O)6}12
{Mo2O4(ligand)}30]n-
(diameter ca. 3nm)
|
Highlighting the application in a beautiful way (see also Consequences) |
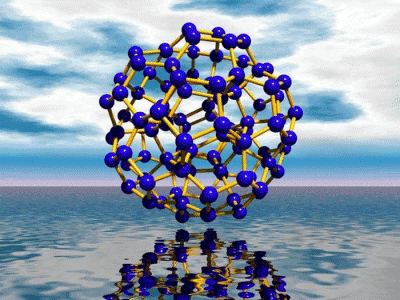
The Keplerate
For the highlight see: Chemical Science, Vol. 5, Issue 3, p. 18 (2008) |
|
Uptake and release of ions |
Closing and opening of the pores having crown-ether function with plugs/guests |
Metal-oxide based bricks from a
"Chemical LEGO® Box" can be used to build an enormous variety of unusual molecular entities.
If for instance five-cornered building blocks of the type {(Mo)Mo5} are used and linked
appropriately it is possible to
construct - according to the known geometrical "Aufbau principle" of a soccer ball
having 12 pentagons - spherically shaped porous molecular nanocontainers. (The structure type shows similarities
to that of the most simple spherical viruses and the geodesic domes.) Remarkably, the 20 pores can
be opened and shut with plugs as
required. This allows to encapsulate and transport materials
which can be released again whenever and wherever needed.
The above mentioned capsules
interact specifically with their environment, e.g. with cations and guests closing the pores (pictured).
One might call them formally(!) artificial
cells as they can be used to mimic some properties of biological cells, like (counter) ion transports
through membrane channels, as well as cells' responses. With a special physical method - Nuclear
Magnetic Resonance spectroscopy -
it is possible to "observe" how the ions pass through the pores/channels with the
intention to study phenomena like ion-separation and dynamics on a nanoscale.
Highlights:
M. Freemantle, "Artificial Cells Allow Ion Entry: Porous Inorganic Capsules Serve as
Model for Biological Ion-Transport Processes", Chem & Eng. News 83/48, Nov. Issue p. 10 (2005)
(http://pubs.acs.org/cen/news/),
L. Cronin, "Inorganic Molecular Capsules:
From Structure to Function",
Angew. Chem. Int. Ed. 45, 3576 (2006), and M. Zgraggen, "Artificial cells mimic ion transport",
Chemistry World, March Issue, 2006, p. 30 (see also Chemical Science, Vol. 5, Issue 3, p. 18 (2008)).
Beauty and Symmetry Relation: The spherical capsules
show interpenetrating
reciprocal solids
spanned by different sets of equivalent atoms.
A collection of all six solids (two Platonic and four distorted(!) Archimedean) is shown, too.
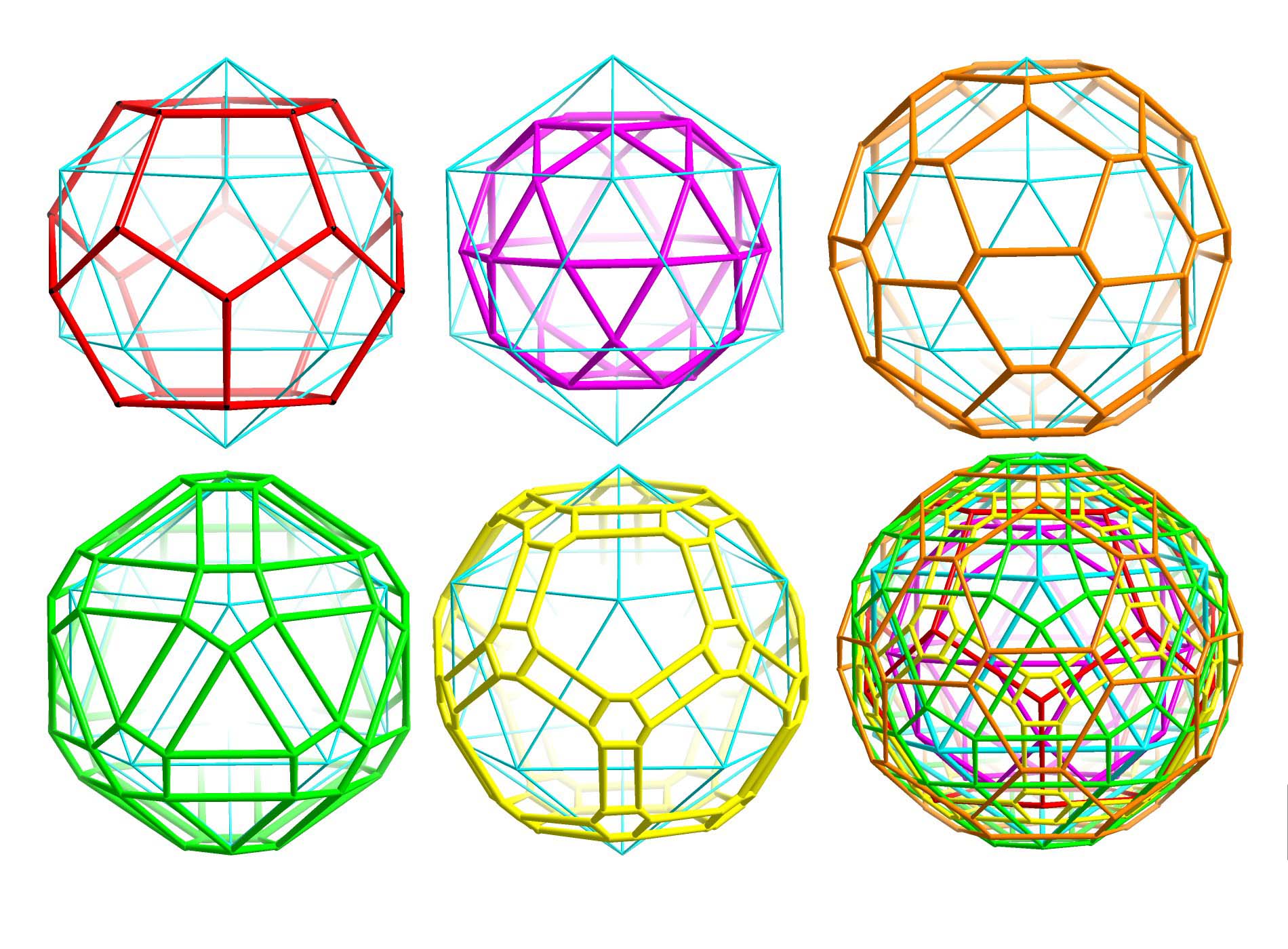
Reciprocal polyhedra as "building units" in
Mo132 related to the icosahedron
For details see publication: A. Müller, "The Beauty of Symmetry", Science 300, 749 (2003) and website of the
American Mathematical Society, Sept. 8, 2003.
http://www.ams.org/
Chemistry Under Confined Conditions (Nanochemistry):
It is possible to perform chemistry on the surfaces,
in the pores, and in the cavities of the capsules (see below).
Research based on the capsules of the type Mo132
shows new routes related to the following topics (examples):
- Unprecedented Nano"Materials": These can be generated in the
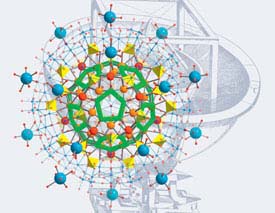
capsule cavities, for example a variety(!) of nanodrops of water with and without
electrolytes. One encapsulated
{H2O}100 nanodrop for instance shows three shells of Platonic and Archimedean solids
(Figure left). Importantly, if the internal capsule cavity is increased
- by replacing larger by smaller internal ligands -
we find the 100 water assembly diluted in an (underoccupied) four-shell system,
i.e. with lower density.
These findings were highlighted many times.
|

{H2O}20{H2O}20{H2O}60
shells encapsulated |
{H2O}100
(result from an X-ray crystallographic study) |
|

|
Higher and lower density confined water (pictured)
"It is one of
water's eccentricities that confinement in narrow spaces can alter its
character profoundly".
P. Ball, A Biography of Water; this fact is of importance for biological processes. |
- Nano-Ion Chromatograph - Nanoscaled Cation Separation:
The capsules can be used to separate different uptaken cations by
placing them on different well defined positions. This is
highlighted as "Traps for cations", Nature Materials
(News & Views) 2, 780 (2003).
- Reactions Inside a Porous Nanocapsule: In the cavities of the functionalized,
porous capsules reactions with the internal ligands -
positioned at the internal shell surfaces - can be performed. An example is the
deliberate aquation/hydration and deprotonation reaction at the linker
fragments {(Mo2O4)C2O4H}+ which influences
the structure of the water/electrolyte encapsulate
.
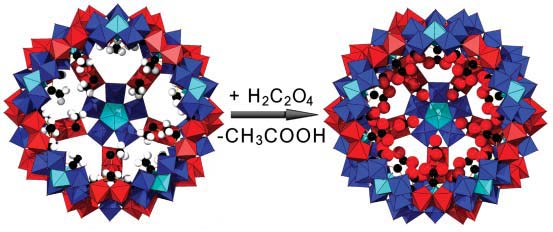
Schematic demonstration of how the inner surface
of the porous spherical nanocapsule can be changed, e.g. by replacing acetate (left)
by oxalate ligands (right) having reactive sites as the second dicarboxylate groups are free
(right); for clarity, one pentagonal unit and five linkers are
omitted ({(Mo)Mo5} not coordinated units (blue/cyan) and {MoV2} type
linkers (red) in polyhedral representation, C black, H white).
Publication: "Reactions inside a porous nanocapsule/artificial cell:
encapsulates' structuring directed by internal surface deprotonations",
Chem. Commun., 3396 (2006)
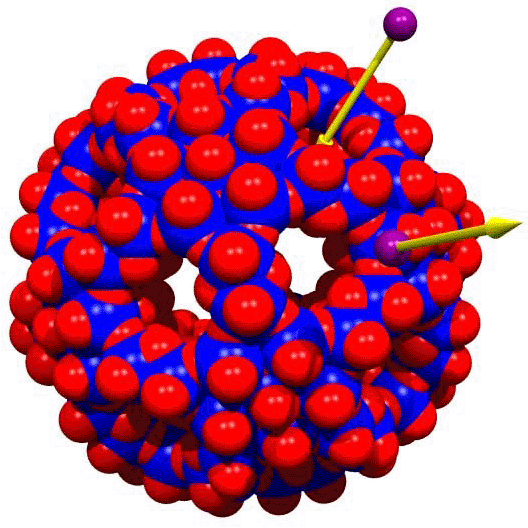
- Confinement Effects and Generation of a Hydrophobic Cavity:
The encapsulation of a large assembly of organic species - the 24 butyrate
unit (pictured below) - exibits a remarkable central spherical hydrophobic
cavity spanned by 72 H atoms. Interesting interactions are found
between the butyrates under the
confined conditions.
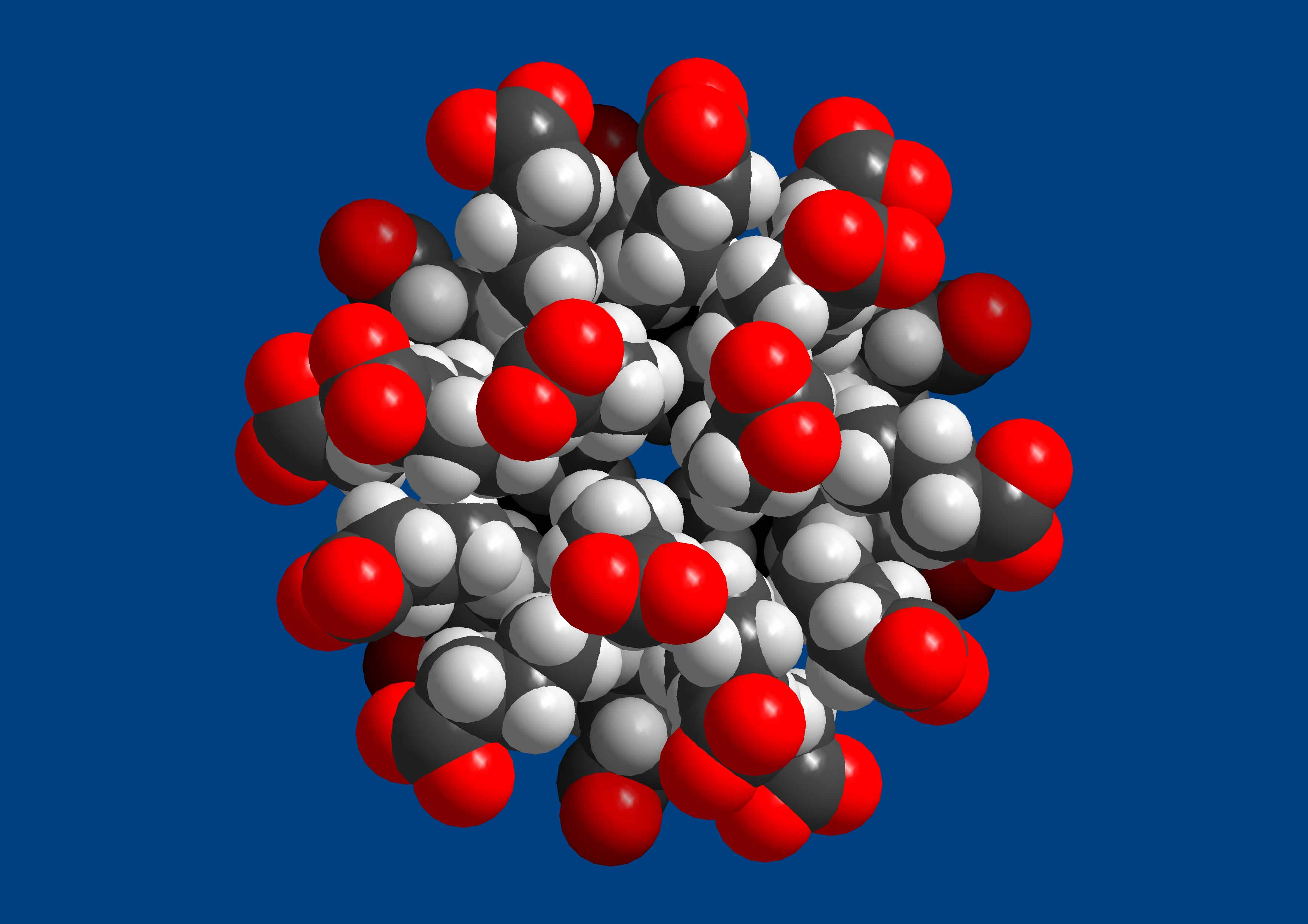
Publication: "A Spherical 24 Butyrate Aggregate with a Hydrophobic Cavity
in a Capsule with Flexible Pores: Confinement Effects and
Uptake-Release Equilibria at Elevated Temperatures", Angew. Chem. Int. Ed.
48, 8051, (2009).
- Pores Maintain Flexible Attitude:
It could be proven that species like carboxylates can enter the capsule even
through smaller pores without changing the
capsule structure irreversibly.

Publication: A. Ziv, A. Grego,
S. Kopilevich, L. Zeiri, P. Miro, C. Bo, A. Müller, I. A. Weinstock,
"Flexible Pores of a Metal Oxide-Based Capsule Permit
Entry of Comparatively Larger Organic Guests",
J. Am. Chem. Soc. 131, 6380 (2009).
Highlighted for example in: S. A. Borman,
"Inorganic Pores Maintain Flexible
Attitude: Molybdenum oxide-based capsules have flexible
pores that allow compounds larger than the pores to
enter and possibly react", Chem. & Eng. News,
April 6, Vol. 87, No. 14, 26 (2009).
- Coordination Chemistry Inside the Cavities as well as in and above the Pores:
The pores with crown-ether function can be closed by guests in a supramolecular fashion (see above).
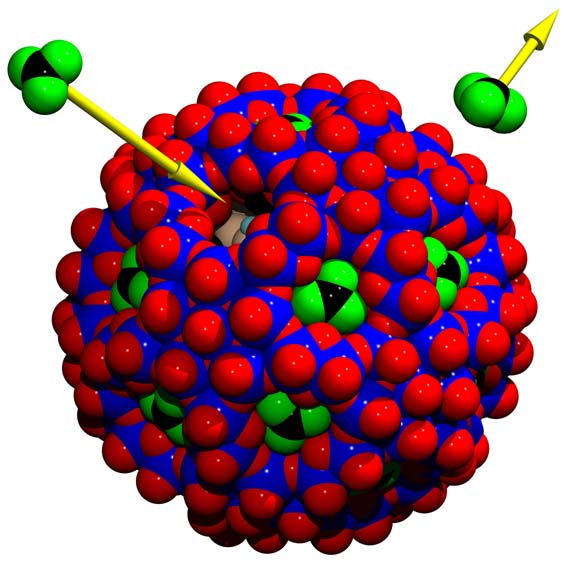
Uptaken smaller cations Mn+ (e.g. M = Ca2+, Ce3+) show
well defined coordination polyhedra (see Nanoion
Chromatograph above) whereas coordination polyhedra of not uptaken cations of the type
[M(H2O)n]n+ can be found above the pores fixed via hydrogen bonds.
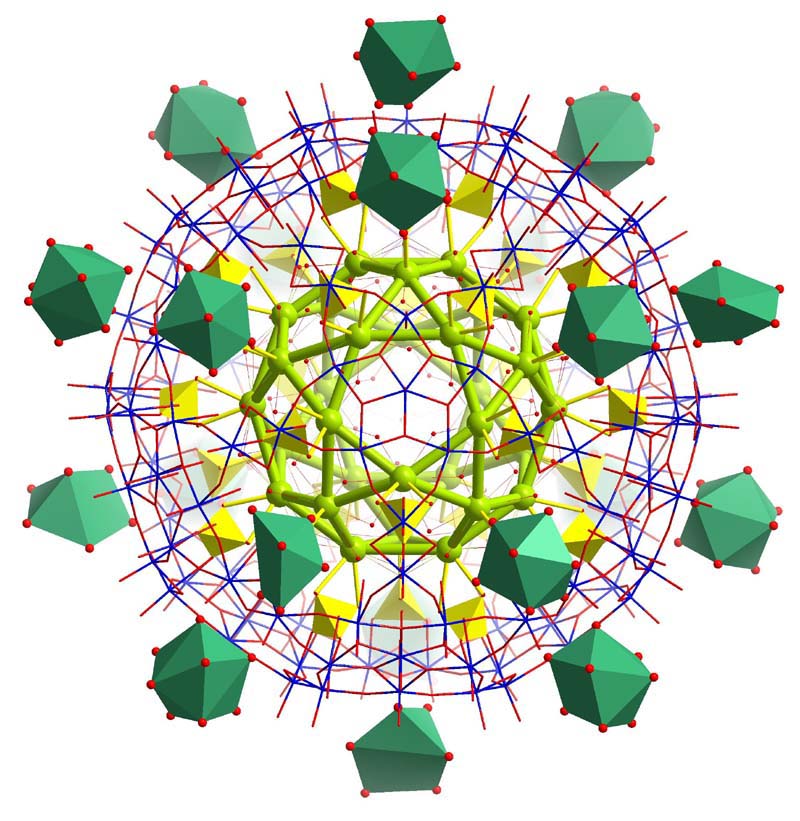
Five internal Pr3+ are disordered over sites
defined by the vertices of the green icosidodecahedron
and external [Pr(H2O)9]3+ complexes are shown as green polyhedra.
Publication: ""Gating" the Pores of a Metal Oxide Based Capsule: After
Initial Cation Uptake Subsequent Cations Are
Found hydrated and Supramolecularly Fixed above the Pores", Angew. Chem.
Int. Ed. 45, 460 (2006) and highlight: M. Gross, "Molecular guests stay at the gates", Chemistry World
Vol. 3, No. 2, 16 (2005); see also: "Cation behavior at an artificial cell interface:
binding distinguished by ion hydration energetics and size", Chem. Commun., 948 (2008).
- Chemistry and Kepler's Early Model of the Solar System:
The term Keplerate was defined around ten years ago by us to describe
structures that contain Platonic and Archimedean solids
one inside another, like Russian dolls.
It honours Johannes Kepler, who in the sixteenth century described
his early model of the cosmos ("Mysterium Cosmographicum") in which the radii of the successive
planetary orbits are proportional to the radii of spheres that
are successively circumscribed around and inscribed within the
five Platonic solids. The term Keplerate is now used for other compounds with shell structure,
too (see for instance Nature, 447,1035 (2007) and
Wiktionary: http://en.wiktionary.org/wiki/Keplerate).

Mo132 showing the
Mo12 icosahedron and Kepler's early cosmos model
(II) Smaller Capsules
If the above mentioned pentagonal
units of the type {(Mo)Mo5} are connected by smaller, i.e. mononuclear linkers M3+
(M = Fe3+, Cr3+) - compared to the case of the Mo132
capsule with binuclear linkers - smaller
capsules are obtained, which show interesting properties, too
(see also "Predicting a structured future", Nature Chemistry 1, 13 (2009)).
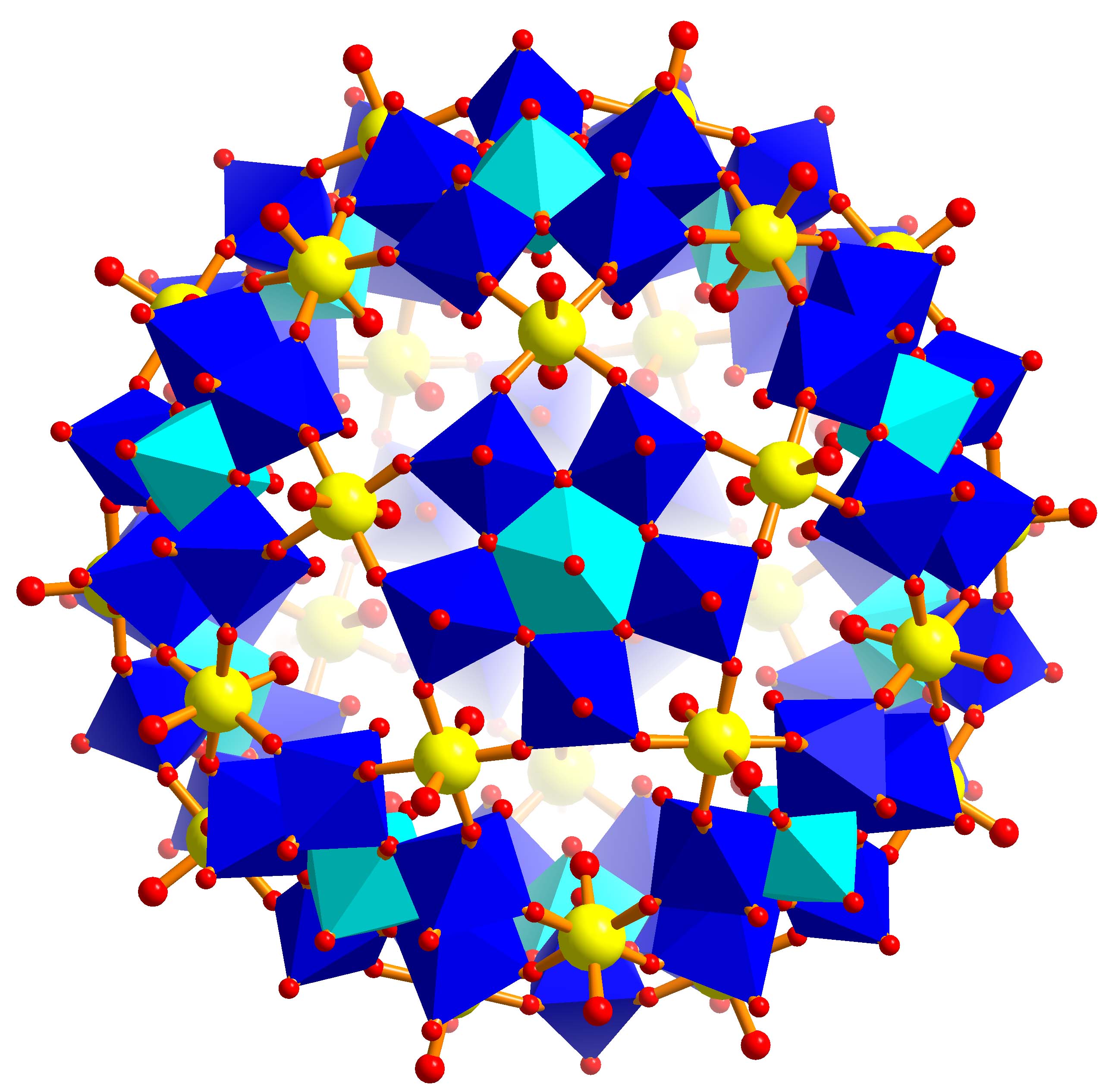
M30Mo72 = M30
{(Mo)Mo5}12
(M = W as yellow spheres)
- Novel Magnetic Materials: If we refer to transition metal
centers M3+ (M = Cr, Fe) as linkers which form an icosidodecahedron
unprecedented molecular magnets can be obtained (see for instance: D. Gatteschi,
R. Sessoli, J. Villain, Molecular Nanomagnets, Oxford Univ. Press, Oxford, 2006 as well as our homepage
with keyword
Molecular Magnets).
- "Chemical Darwinism": The formation tendency of the spherical
molybdenum oxide based
capsules is so high that they are formed from fragments obtained by reaction of metal
cations M3+ with simple molybdenum oxide
clusters. Important: the nondecomposed clusters
get integrated/protected in the capsules, i.e. are adapted to the system
(Chem. Commun. 657 (2001); see also highlight: J.-M. Lehn, Chem. Soc. Rev. 36,
151 (2007) referring in the context to the sentence "The supramolecular
organisation drives the selection of the
components giving the "fittest" constituent" related to
footnote 60).
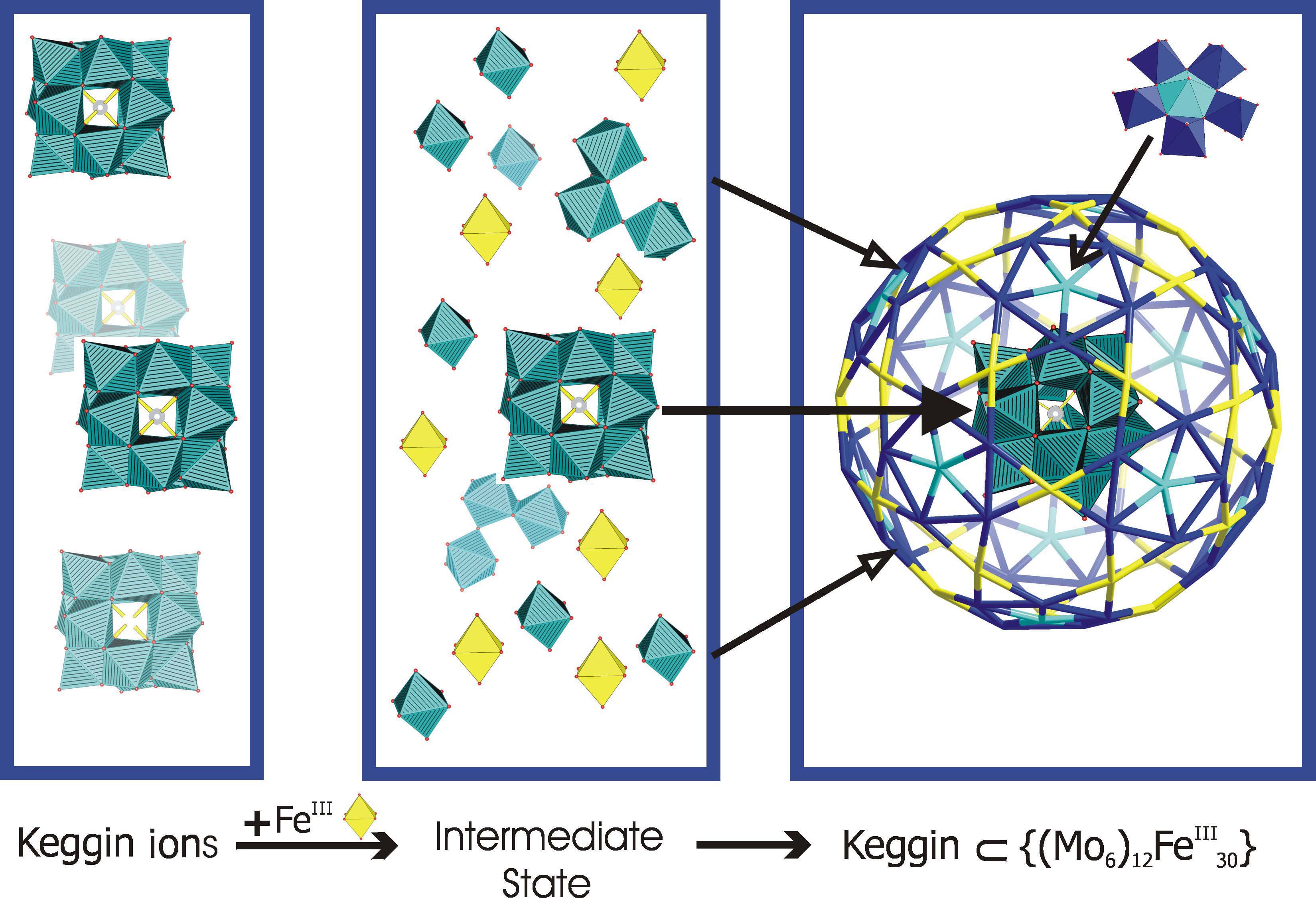
- Assembly of the Capsules: The capsules form in aqueous solution
- based on a new type of assembly process - giant
spherical aggregates with diameters of up to 100 nm (basic principles of the type of process
are presented in J. Chem. Education
84, 526 (2007); for further interesting results, see also
Consequences)
- Sphere Surface Supramolecular Chemistry: The 20 {Fe3M3O6}
pores can be closed, e.g. with 20 NH4+ ions
which can be partly released in solution dependent on the type of solvent. A challenge will be to study,
based on the stepwise fixation of appropriate substrates/guests
effects related to allostery,
cooperativity, and regulation - in other words, to study interrelated
features which result when the occupation of a given site leads to
changes in the properties of the other sites.
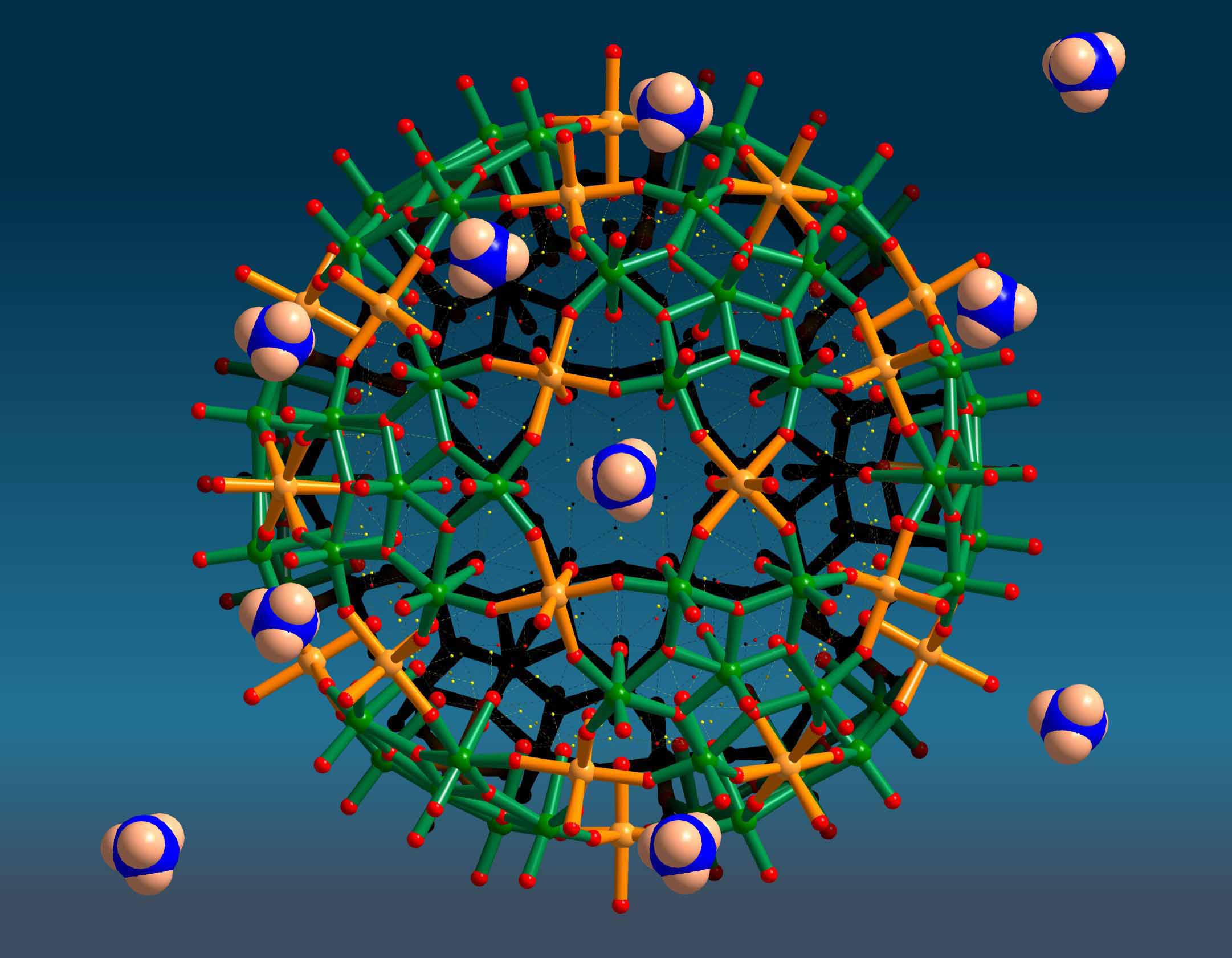
Publication: " Porous Capsules
{(M)M5}12FeIII30 (M = MoVI, WVI):
Sphere Surface Supramolecular Chemistry with 20 Ammonium Ions,
Related Solution Properties, and Tuning of Magnetic Exchange Interactions",
Angew. Chem. in press.
Detailed Literature:
Research Overview and P. Gouzerh, M. Che,
"From Scheele and
Berzelius to Müller: Polyoxometalates (POMs) revisited and the "missing
link" between the bottom up and top down approaches",
L'Actualité Chimique
June Issue, No. 298, 9-22 (2006), the Feature Article: A. Proust,
R. Thouvenot, P. Gouzerh, "Functionalization of polyoxometalates: towards advanced
applications in catalysis and materials science", Chem. Commun. 1837-1852 (2008)
in the sections "Introduction" and "Nanosized polyoxomolybdates
with multifunctionality" as well as L. Cronin, "High
Nuclearity Clusters: Iso and Heteropolyoxoanions and Relatives", in Comprehensive
Coordination Chemistry II, Vol. 7 (Volume Editors: M. Fujita, A. Powell, C. A Creutz),
Elsevier, Amsterdam, 2004, pp. 1-56.

















