Not updated. See list of publications (Chronological) and other topics of Research.
The information below highlights one of our key continuing
research projects (see Selected Papers), and is presently our main one.
For authors' names of the given papers, see list of publications.
Some earlier reviews on basic chemistry:
- "Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines",
Angew. Chem. Int. Ed. Engl. 30, 34-48 (1991)
- "A variety of combinatorially linkable units as disposition: from a giant
icosahedral Keplerate to multi-functional metal-oxide based network structures",
Chem. Comm. 1347-1358 (1999)
- "Polyoxometalates: Very Large Clusters - Nanoscale Magnets",
Chem. Rev. 98, 239-271 (1998)
- "A variety of combinatorially linkable units as disposition:from a giant
icosahedral Keplerate to multi-functional metal-oxide based network structures",
Chem. Comm. 1347-1358 (1999)
- "Soluble Molybdenum Blues - 'des Pudels Kern'", Acc. Chem. Res. 33, 2-10 (2000)
In the below listed original papers, it was argued that only the molybdate
system allows the described versatile chemistry.
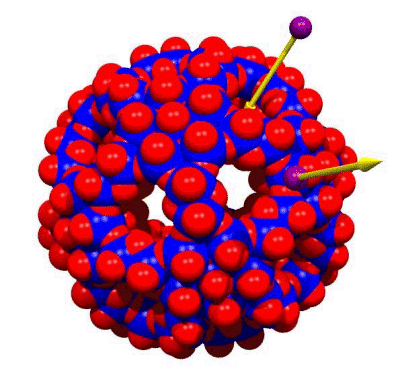
From Porous Capsules/Artificial Cells to Sphere Surface and
Super-Supramolecular Chemistry: Perspectives for Chemistry under
Confined Conditions, Artificial Cell Environment Interactions as well as Nanotechnology
 |
 |
| One of the
20 Mo9O9
pores with crown ether function |
{Mo132}
Structurally well-defined porous spherical metal-oxide based
nanocapsules/artificial cells of the type {Pentagon}12{Linker}30
allow unprecedented chemistry (Figure). The area shows revolutionary
routes to different disciplines, like chemistry (e.g. modelling passive
ion transport through membranes), materials science (e.g. construction of
a nano ion chromatograph), physics (especially regarding confined matter
properties), and mathematics (concerning the tiling problem of spherical
surfaces).
This is based on the following related facts:
the size of the capsules and their pores/channels can be
tuned, while the latter can be opened and shut,
the internal cavity's shell functionality can be tuned,
e.g. from hydrophilic to hydrophobic, and
the twenty(!) abundant pores of the nanosponge have crown-ether
functions with respect to cations allowing sphere-surface
as well as super-supramolecular chemistry including in
principle the study of allosteric effects.
Options for the following basic/new type of research topics are evident:
Encapsulation Chemistry: This refers to studies of encapsulated
nanomaterials of different types - including water with and without
electrolytes - taken up through capsule pores. The capsules themselves
can separate/position cations like a nano ion chromatograph in the sense
of structure directed vectorial-type translocations.
Capsule-Coordination Chemistry: This allows a new type of
spectroscopic and magnetic studies of encapsulated discrete/shielded
mono- and polynuclear complexes, while encapsulated water shells
can act as polydentate ligands.
Artificial Cell Environment Interactions: In this respect
some of Nature's pathways can be modelled, like ion uptake-and-release
processes (Figure) as well as cell response to stimuli, since pore
closing by molecules like corks - formally considered
as "stimuli" - influences significantly encapsulates' (like water)
structures; this refers to confined condition phase transfer. Generally
speaking, model cell environment interactions
can be studied on a wide range.
- "Artificial Cells: Temperature-Dependent Reversible Li+-Ion Uptake/Release
Equilibrium at Metal Oxide Nanocontainer-Pores", Angew. Chem. Int. Ed. 43, 4466-4470 (2004);
Corrigendum: 43, 5115
- "En route to coordination chemistry under confined conditions in a porous capsule:
Pr3+ with different coordination shells", Chem. Comm. 2038-2039 (2004)
- "Drawing Small Cations into Highly Charged Porous Nanocontainers Reveals "Water"
Assembly and Related Interaction Problems", Angew. Chem. Int. Ed. 42, 2085-2090 (2003)
- "Trapping Cations in Specific Positions in Tuneable "Artificial Cell" Channels:
New Nanochemistry Perspectives", Angew. Chem. Int. Ed. 42, 5039-5044 (2003)
- "Changeable Pore Sizes Allowing Effective and Specific Recognition by a
Molybdenum-Oxide-Based "Nanosponge": En Route to Sphere-Surface and Nanoporous-Cluster
Chemistry", Angew. Chem. Int. Ed. 41, 3604-3609 (2002)
- "Thirty Electrons "Trapped" in a Spherical Matrix: A Molybdenum Oxide-Based
Nanostructured Keplerate Reduced by 36 Electrons", Angew. Chem. Int. Ed. Engl.
39, 1614-1616 (2000)
- "Archimedean Synthesis and Magic Numbers: "Sizing" Giant Molybdenum-Oxide-Based
Molecular Spheres of the Keplerate Type", Angew. Chem. Int. Ed. Engl. 38,
3238-3241 (1999)
Highlighted, e.g. in:
- W. G. Klemperer, G. Westwood, "Traps for Cations",
Nature Materials (News & Views) 2003, 2, 780.
- M. Gross, "Encapsulating Chemistry", Chemistry in Britain 2003, Aug. Issue, p. 18.
- N. Hall, "Bringing Inorganic Chemistry to Life",
Chemical Communications 2003, 803.
- F. Frick, "Das Zauberreich der Moleküle" (with cover picture),
Bild der Wissenschaft 03/2003, 76-98.
- R. Kurschat, "Synthese eines gastfreundlichen Molekülclusters - Erster
Schritt zu einer "super-supramolekularen" Chemie", Neue Zürcher Zeitung, 13.11.2002.
- "Molibdeno con sorpresa", El Pais (Spain), 6. January 1999.
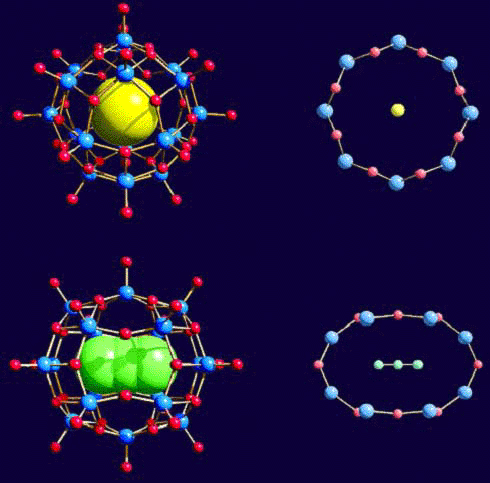
Nanoscaled Objects: Characteristic Internal and
External Surface Functionalities Show Routes for a New Type of Chemistry
|
 |
{Mo12} Ì {Mo72Fe30}
|
Nanoscaled species with well-defined surface-structured landscapes
have properties unknown for small molecules and can give rise (if they can be
dissolved) to a versatile chemistry depending on their special type of
surface functionalities as well as on their molecular shapes. In
the present case this refers to our two important consequential(!) forms,
the spherical hollow- and wheel-type kind.
Options include:
Direct intermolecular surface interactions leading to
unusual assemblies in the gas and solute phase.
Unprecedented solvent structuring at their surfaces - especially in the case of water (see below).
Placing different groups - including multiphilic species (see paper: "On the option of generating ...")
- at different positions, which can lead to unusual surfaces
and structures (see Figure left as well as the first cited paper).
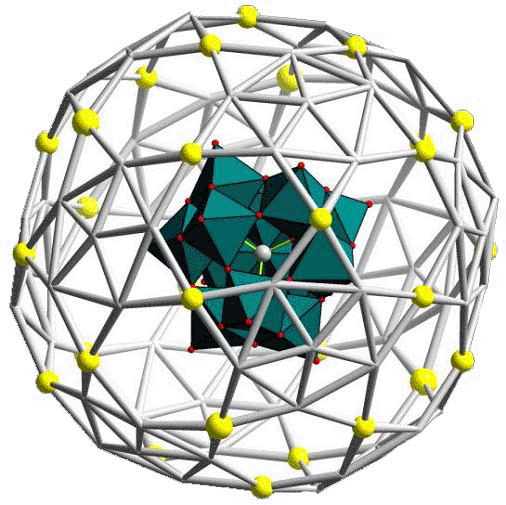
Reactions/interactions at external as well as internal surface
positions of both the covalent and non-covalent type. This even
allows the fixation/encapsulation of a cluster inside a cluster
cavity based on non-covalent interactions leading to a new type of
supramolecular compound (Figure right).
Cross-linking in a solid state reaction at room temperature in case of
the abundance of highly reactive surface functionalities
(like Fe(H2O)3+).
Metal-oxide based nucleation under confined conditions, starting with
covalent linking from internal surface sites, as in case of molecular wheel
cavities (last paper).
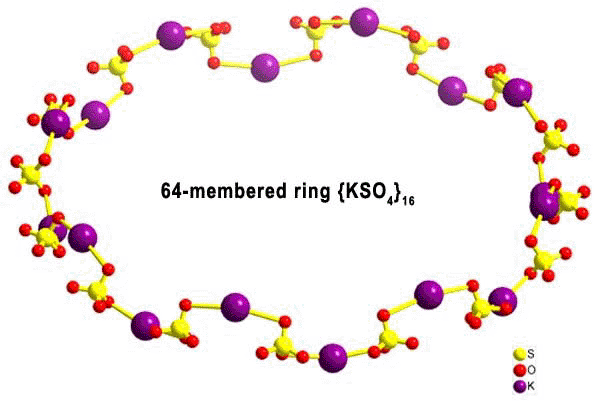
- "Synergetic activation of "silent receptor" sites leading to a new type
of inclusion complex: integration of a 64-membered ring
comprising K+ and SO42- ions
into a molybdenum oxide-based nanoobject", Chem. Comm., 2000-2001 (2003)
- "Paramagnetic Keplerate "Necklaces" Synthesized by a Novel Room-Temperature
Solid-State Reaction: Controlled Linking of Metal-Oxide-Based Nanoparticles", Angew.
Chem. Int. Ed. 41, 579-582 (2002)
- "'Molecular Symmetry Breakers' Generating Metal-Oxide-Based Nanoobject
Fragments as Synthons for Complex Structures:
[{Mo128Eu4O388H10(H2O)81}2]20-, a
Giant Cluster Dimer", Angew. Chem. Int. Ed. 41, 2805-2808 (2002)
- "Soccer-playing metal oxide giant spheres: a first step towards patterning
structurally well defined nano-object collectives", Chem. Comm., 440-441 (2002)
- "Urea as 'deus ex machina' in giant molybdenum blue type cluster synthesis: an
unusual hybrid compound with perspectives for related nano, supramolecular
and extended structures", Chem. Comm., 2000-2002 (2002)
- "'Nanoobjects' by Self-Assembly Concomitant with Modifications under Alterable
Boundary Conditions: Incorporation of Paramagnetic Metal Centers (Cu2+) in
Ring-Shaped Molybdenum-Oxide Based Clusters", Angew. Chem. Int. Ed. 40, 4034-4037 (2001)
- "On the option of generating novel type surfaces with multiphilic ligands
wihtin the cavity of a giant metal-oxide based wheel type cluster: chemical
reactions with well-defined nanoobjects", Chem. Comm., 655-656 (2001)
- "Generation of cluster capsules (Ih) from decomposition products of a
smaller cluster (Keggin - Td) while surviving ones get encapsulated: species
with core-shell topology formed by a fundamental symmetry-driven
reaction", Chem. Comm., 657-658 (2001)
- "Linking Icosahedral, Strong Molecular
Magnets {MoVI72FeIII30} to Layers - A Solid-State
Reaction at Room Temperature", Angew. Chem. Int. Ed. Engl. 39, 1612-1614 (2000)
- "A New Type of Supramolecular Compound: Molybdenum-Oxide-Based Composites
Consisting of Magnetic Nanocapsules with Encapsulated Keggin-Ion Electron
Reservoirs Cross-Linked to a Two-Dimensional Network", Angew. Chem. Int. Ed. 39,
3413-3417 (2000)
- "Assembling nanosized ring-shaped synthons to an anionic layer
structure based on the synergetically induced functional complementarity of
their surface-sites: Na21[MoVI126MoV28O462H14(H2O)54(H2PO2)7] . x H2O (x = ca. 300)",
Chem. Comm., 1035-1036 (1999)
- "Molecular growth from a Mo176 to a Mo248 cluster",
Nature 397, 48-50 (1999)
Highlighted, e.g. in:
- D. A. Schiraldi, "An Unusual Inorganic 64-Membered Ring", "Heart Cut"
(website of the American Chemical Society), 8.9.2003.
- G. R. Desiraju, "Chemistry beyond the molecule", Nature 2001, 412, 397-400.
- J. Uppenbrink, "A Soupçon of Phosphate", Science (Editors' Choice) 2000, 290, 411.
- A. Gerhard, "Molekulare Riesenräder verknüpft", Frankfurter Allgemeine
Zeitung, 24. September 1997.
Dynamical Library Building Units Number-Increase
Can Lead to Ever Larger Nanoscaled Assemblies: Symmetry Breaking at
a Giant Cluster Surface
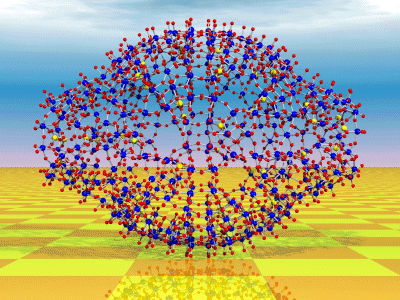
{Mo368}
The nanocosmos as such, does not show the variety-limiting translational
symmetry restriction of macroscopic crystalline materials but offers - in
contrast to the "microcosmos" with its small molecules - the possibility
that (several) larger arrays with local symmetries differing from the
overall symmetry occur, a situation well known for spherical viruses and
which increases the option for the generation of an extreme structural
variety tremendously. Thus the appropriate building units must display a
certain type of flexibility as prerequisite for linking, a condition
well-fulfilled by molybdenum-oxide based fragments/aggregates under
reducing conditions (i.e., those occurring in molybdenum blue solutions).
These fragments abundant as potential ingredients of a "dynamic library",
can, by a type of "split-and-link process", adapt their size and shape
dependent on slight alterations of the relevant boundary conditions which
can lead to the formation of a variety of unusual giant molybdenum-oxide
based clusters. A unique example corresponds to the formation of a cluster
with the size of hemoglobin (diameter approximately 6 nm), which contains
1880 non-hydrogen (368 metal) atoms. It is formed by the linking of
64 {Mo1}-, 32{Mo2}-, and 40{Mo(Mo5)}-type
units (exhibiting different
stoichiometries) according to a remarkable symmetry-breaking process,
which is nicely recognizable at the cluster surface (see Figure;
Mo blue, O red, S yellow).
- "On the complex hedgehog-shaped cluster species containing 368
Mo atoms: simple preparation method, new spectral details and
information about the unique formation", Polyhedron 23, 2381-2385 (2004)
- "Inorganic Chemistry Goes Protein Size: A Mo368 Nano-Hedgehog
Initiating Nanochemistry by Symmetry Breaking", Angew. Chem. Int.
Ed. 41, 1162-1167 (2002)
Highlighted, e.g. in:
- Encyclopaedia Britannica, 2004. Encyclopaedia Britannica
Premium Service. "Mathematics and Physical Sciences". 6.
November 2004 (http://www.britannica.com/eb/article?tocld=91842).
- R. Kurschat, "Ein Igel im Nanoformat: Molybdän-Cluster erreicht
die Größe von Eiweißmolekülen", Neue Zürcher Zeitung, 15.5.2002.
- M. Sokolov, V. Fedin, "Record in Inorganic Chemistry", Nauka v
Sibiri (Science in Siberia), Issue 2358-2359, June 2002.
- M. Gross, "Molybdates make a nano-hedgehog", Chemistry in
Britain, 2002, 38, 16.
- P. Hergersberg, "Deutsche Chemiker bauten das größte Molekül", Die Welt, 23.5.2002.
- R. Kurschat, "Ein Igel im Nanoformat: Molybdän-Cluster erreicht die Größe von
Eiweißmolekülen", Neue Zürcher Zeitung, 15.5.2002.
- P. Ball, "The blue lemon", Nature, materials update, 25.4.2002.
- D. Bradley, "Nano the Hedgehog", The Alchemist, News Research, 18.4.2002.
- "Winzige Riesenkugel", Die Zeit, 8.5.2002.
- S. Stinson, "Molybdenum blue puzzle may be solved", Chemical &
Engineering News, 3.6.1996.
Confined Water and the Mystery of the Liquid
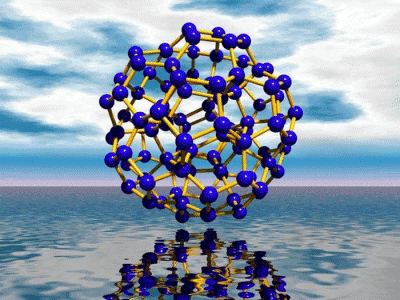
{H2O}100
The properties and structure of "The Liquid Water" including its
behaviour as a function of temperature and/or pressure as well as
in the presence of (poly)electrolytes are yet not well understood.
An important investigation, particularly for an understanding of
several aspects of the role of water in biological processes,
would be to study how moderately large assemblies of water
molecules - say from 10 to 1000 - respond to their confinement
in nanometer-sized cavities. (Note: In biological cells the
water between biomolecules consists only of a rather small
number of layers of molecules.) In the context of our work
on "Encapsulation Chemistry" it is possible to influence
encapsulated water-assembly structures on a wide range by the
size, charge, internal surface, and encapsulated electrolytes
as well as the capsule-hosts. The obtained information also provides
general knowledge about the intrinsic properties of water molecules
in general as well as about their potentiality to aggregate stepwise
under confined conditions. The largest water cluster obtained consists
of 100 H2O molecules (Figure).
- "Chameleon Water: Assemblies Confined in Nanocapsules", J. Mol. Liquids (in press)
- "Structure of a cavity-encapsulated nanodrop of water", Inorg.
Chem. Comm. 6, 52-53 (2003)
- "Drawing Small Cations into Highly Charged Porous Nanocontainers
Reveals 'Water' Assembly and Related Interaction Problems", Angew.
Chem. Int. Ed. 42, 2085-2090 (2003)
Highlighted, e.g. in:
- G. Zosimo-Landolfo, "À la découverte de l'eau",
Biofutur 2003, Feb. Issue, p.17.
- R. Kurschat, "Wasser in Gefangenschaft - Winzige Käfige
helfen bei der Analyse von Molekülclustern", Frankfurter
Allgemeine Zeitung, 3.11.2003.
- N. Izarova, V. Fedin, "Nanodrop of water in the giant
sperical cluster", Nauka v Sibiri (Science in Siberia),
Issue 2386, December 2002.
Unprecedented Vesicle Formed From
Wheel-Shaped-Type Clusters Exhibiting Hydrophilic Surfaces: A
New Solute State of Inorganic Ions
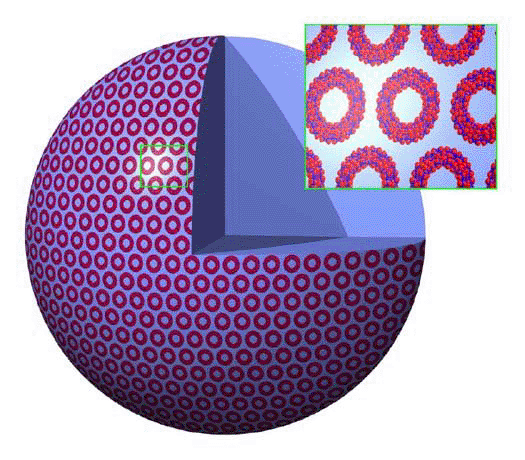
Special kinds of molecules abundant in solution can aggregate
to different kinds of superstructured hierarchies: surfactants
and membrane lipids can assemble into complex structures such as
micelles, liposomes, or hollow vesicles due to their amphiphilic character.
The wheel-shaped clusters, isolated from molybdenum blue solutions,
with their hydrophilic surfaces and related high solubility, like
the "classical" Mo154, form in aqueous solution an unprecedented
type of (hollow) superstructure/vesicle, where ca. 1200 molecular
wheels form a sphere (80 nm diameter) with an encapsulated nanodrop
of water (Figure). According to the rather large hydrophilic cluster
surface, the interface water gets structured (proven by dielectric
spectroscopy), which probably contributes
to the vesicle formation, and may be described also by a new
inorganic ion solute state. The high solubility prevented the isolation
of crystals from Mo blue solutions for more than 200 years, since the first experiments were done by
Scheele and Berzelius
- "Self-assembly in aqueous solution of wheel-shaped
Mo154 oxide clusters into vesicles", Nature 426, 58 (2003)
- "Hierarchic patterning: architectures beyond 'giant
molecular wheels'"
Chem. Comm., 1928-1929 (2001)
Highlighted, e.g. in:
- "Rounding up nanoclusters - nanotechnology", Materials
Today 2004, Jan. Issue, p. 10.
- "Hohlkugel aus Riesenrädern", Spektrum der
Wissenschaft 2004, Jan. Issue, p. 9.
- R. Kurschat, "Eine anorganische Haut für Wassertröpfchen", Neue
Zürcher Zeitung, 19.11.2003.
Icosahedral Nanocapsules' Interpenetrating
Platonic and Archimedean Solids: Chemistry and Aesthetics
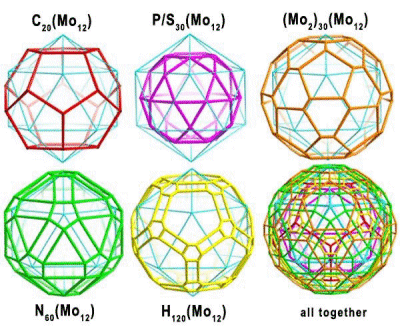
As the capsules of the type {Pentagon}12{Linker}30 have the
highest possible (icosahedral) symmetry for molecules, a large
number of sets of equivalent integrated as well as encapsulated
atoms/species (like water molecules) are automatically generated,
which span several more or less distorted Platonic and Archimedean solids.
The architectural variety of such interpenetrating solids even fascinates
mathematicians. The complex cluster system
[{(NH2)3C+}20 +
({H2O}20 Ì {H2O}20 Ì {H2O}60)
Ì
{(Mo)Mo5O21(H2O)6}12
{Mo2O4(SO4/H2PO2)
2}30]32- shows,
e.g. the
following solids: an icosahedron (blue), dodecahedron (red), icosidodecahedron
(violet), truncated icosahedron (brown), rhombicosidodecahedron (green),
and rhombitruncated icosidodecahedron (yellow) spanned by 12 Mo, 20 C,
30 S/P, 30 Mo2, 60 N, and 120 H, respectively (Figure).
Surprisingly, the ratio of edges of the 30 rectangles of
the {H2O}60 shell is very near to the value of the 'golden
section' (i.e., ca. 1.6). In this sense the Platonic philosophy
of beauty, truth and good can be referred to - if one also takes into
account the importance of water for our, and all organisms' daily life
in the context of the statements of Thales and Paracelsus.
- "The Beauty of Symmetry", Science (Perspectives) 300, 749-750 (2003)
Highlighted, e.g. in:
- R. Dagani, "Like a fullerene, but no carbon", Chem. & Eng. News 2003, 81, 13.
- T. Phillips, S. Brook, "Beyond Buckyballs", American Mathematical
Society, July 2003 (referring to our publication on "The Beauty
of Symmetry", Science 2003, 300, 749).
Templates Direct Like Conductors Fragment Linking
 |
Halide as template
|
| Azide as template |
During the generation of giant polyoxometalate clusters template-directing
processes are often involved. A nice text-book example refers to
the controlled linking of O=VO4 fragments. The templates
are either added to the reaction mixture or are formed in it as "seedlings";
they regulate the mobility of
the basic building blocks in solution and guide, like a conductor,
the usually freely mobile fragments into a well-defined order.
A comparison with the term "slaving principle" in the terminology of Hermann
Haken's Synergetics is certainly worthwhile. In the present case, the finally
formed system is of the host/guest type (figured) where the host is complementary to the
guest/template which is encapsulated.
Unusual Molecular Anion Cages: "The Taming of the Shrew"
(special aspect; see paper below with that title)
The properties of ions in the solid state as well as in solution can in general only be
evaluated in the presence of appropriate "perturbing" counterions being necessarily present due to the
governing principle of electroneutrality. Therefore, it is very difficult
to derive the properties of the free ions from spectroscopic data.
However, this dilemma can be avoided by encapsulating anionic
species as guests within a rigid host that is
also negatively charged, for example a hollow isopolyvanadate, as mentioned above. This leads to
relatively weak interactions, and hence, to rather large distances between
the anions and the cluster shell. The resulting pseudomechanical fixation of
the guests by the host offers the opportunity to have an almost isolated anion in a cryptand
as object of scientific curiosity. The "corresponding
counterions" in this case are located
far away, outside the host-guest system, that is in the cationic lattice and
have, therefore, no noticeable effect on the encapsulated anions.
- "Topologically Interesting Cages for Negative Ions with Extremely
High "Coordination Number": An
Unusual Property of V-O Clusters",
Angew. Chem. Int. Ed. Engl. 29, 926-927 (1990)
- "Formation of a Cluster Sheath around a Central Cluster
by a 'Self-Organization Process': the Mixed Valence
Polyoxovanadate [V34O82]10-",
Angew. Chem. Int. Ed. Engl. 30, 588-590 (1991)
- "[AsVVV12VIV2O40]7-, A
Topologically Interesting Mixed
Valence Cluster as Model for Vanadium Minerals Formed by Weathering",
Angew. Chem. Int. Ed. Engl. 30, 210-212 (1991)
- "Induced molecule self-organization", Nature 352, 115 (1991)
- "Topologisch und elektronisch bemerkenswerte "reduzierte" Cluster des Typs
[V18O42(X)]n- (X = SO4, VO4) mit
Td-Symmetrie und davon abgeleitete
Cluster [V(18-p)As2pO42(X)]m-
(X = SO3, SO4, H2O; p = 3,4)",
Z. anorg. allg. Chem.
595, 251-274 (1991)
- "Vanadium(IV) and Mixed-valence Vanadium(IV/V) Oxygen Clusters with Novel
Electronic Properties, including the Examples
[V12As8O40(HCO2)]n- (n = 3 or 5)
", Chem. Commun., 273-274 (1991)
- "[H3KV12As3O39(AsO4)6- and Related
Topological and/or Structural Aspects of Polyoxometalate Chemistry",
Inorg. Chem. 30, 4935-4939 (1991)
- "Template Controlled Formation of Cluster Shells or a Type of Molecular Recognition:
Synthesis of [HV22O54(ClO4)]6- and
[H2V18O44(N3)]5-",
Angew. Chem. Int. Ed. Engl. 30, 1674-1677 (1991)
- "A Novel Host/Guest System with a Nanometer Large Cavity for Anions and Cations:
[2NH4+, 2Cl- in V14O22(OH)4
(H2O)2(C6H5PO3)8]6-",
Angew. Chem. Int. Ed. Engl. 31, 1192-1195 (1992)
- "Control of the Linkage of Inorganic Fragments of V-O Compounds: From
Cluster Shells as Carcerands via Cluster Aggregates to Solid-State Structures",
Angew. Chem. Int. Ed. Engl. 32, 909-912 (1993)
- "Magnetic Properties of Isostructural Dodecanuclear
Polyoxovanadates with Six and Eight Vanadium (IV) Ions",
Inorg. Chem. 32, 2114-2117 (1993)
- "The Taming of the Shrew: Studying the Interaction of Ions with Ionic
Carcerands as Molecular Containers", Naturwissenschaften 80, 77-78 (1993)
- "[As4Mo6V7O39(SO4)]4-:
A Species with an Unusual Structure and a Model for the
Different Host-Guest Properties of Poly-vanadates and -molybdates",
Chem. Commun., 2539-2540 (1994)
- "Modelling the Remote-Controlled Organization of Particles in a
Nanodimensional Cavity: Synthesis and Properties of
(Et3NH)3(tBuNH3)2Na2[(H2O)2, N3- in
V14O22(OH)4(PhPO3)8] . 6H2O . 2DMF",
Angew. Chem. Int. Ed. Engl. 34, 779-781 (1995)
Reviews:
- "Supramolecular inorganic species: An expedition into a fascinating, rather unknown land mesoscopia with
interdisciplinary expectations and discoveries",
J. Mol. Struct. 325, 13-35 (1994)
- "Supramolecular Inorganic Chemistry: Small Guests in Small and Large Hosts",
Angew. Chem. Int. Ed. Engl. 34, 2328-2361 (1995)
- "Polyoxometalates: Very Large Clusters - Nanoscale Magnets", Chemical Reviews 98, 239-271 (1998)
Highlighted, e.g. in:
- P. C. H. Mitchell, "Open and shut case for anions",
Nature, 348 (1990), 15 (News and Views).
- M. T. Pope, "Anion guests in heteropolyanions?", Nature,
355 (1992), 27 (Scientific Correspondence).
- A. Dress, "Dirigenten komplexer Synthesen", Frankfurter
Allgemeine Zeitung, 2. March 1994.
- J. Emsley, "Science: the peculiar properties of molecules in cages", New Scientist, 24. November 1990, p. 26.
- H. Reuter, "New Impetus for Inorganic Host-Guest Chemistry",
Angew. Chem. Int. Ed. Engl., 31 (1992), 1185.
- P. N. W. Baxter, "Metal Ion Directed Assembly of Complex Molecular
Architectures and Nanostructures"; Section: Polyoxometallate (vanadate) Cages, in "Comprehensive Supramolecular Chemistry", Vol. 9
(Eds.: J. L. Atwood, J. E. D. Davies, D. D. MacNicol, F. Vögtle; Vol. Eds.: J.-P. Sauvage, M. W. Hosseini),
Pergamon/Elsevier, Oxford, 1996, 200.
- The unusual host-guest systems (bonding) are highlighted in the paper:
M.-M. Rohmer, M. Bénard, J.-P. Blaudeau, J.-M. Maestre, J.-M. Poblet,
"From Lindqvist and Keggin ions to electronically inverse hosts: Ab initio
modelling of the structure and reactivity of polyoxometalates",
Coord. Chem. Rev., 178-180 (1998), 1019. We read: "The clear identification of a
polyoxoanion as a supramolecular species involving a
host cage and an encapsulated guest therefore introduced
a quite novel, unusual,
and, at first, controversial concept." (p. 1020) and "The topology of
highly charged hosts is not sufficient, however, to stabilize
the guest anion. The presence of countercations generates an upfield shift which
appears sufficient in most cases to ensure the thermodynamic stability of the
host-guest complex." (p. 1048).










