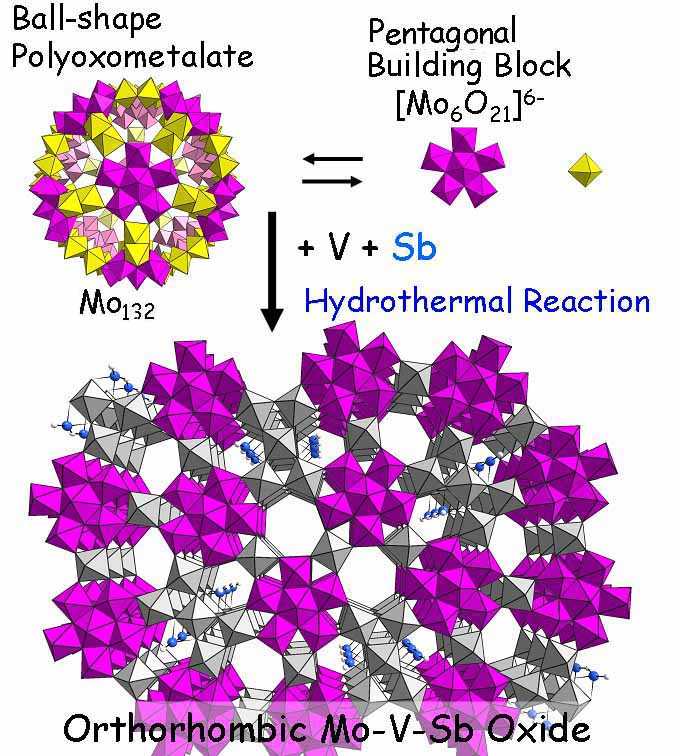

Part of the text:
"Actually, until the beginning of the 1990s, this wide class of compounds
was dominated by a few structural archetypes, namely, the Lindqvist,
Keggin, and Dawson anions. From 1994, Müller has advanced the knowledge of
polyoxometalate chemistry with numerous reports on unprecedented
nanosized arrangements based on molybdenum oxide clusters. These
topological structures are characterized by a striking pentagonal
building unit {Mo(Mo5)}, which condenses with metal cations to form
either spheroidal capsules (keplerate) or wheel-shaped clusters."
Graphical Abstract:
 | Linked to the Pentagon: The addition of molybdate to
[HBW11O19]8- ions leads to the formation of
mixed pentagonal units {W(Mo5)} and {W(WMo4)} trapped as linkers
in the resulting modular assemblies, thus establishing the first link
between the conventional Keggin ions derivatives and the giant molybdenum oxide
and keplerate ions. |
Materials obtained by encapsulation of our metal-oxide species in cationic surfactants; this refers to the construction of nanostructures at interfaces (I), of liquid crystals (II) as well as of LB films and monolayers (III).
Related Publications:

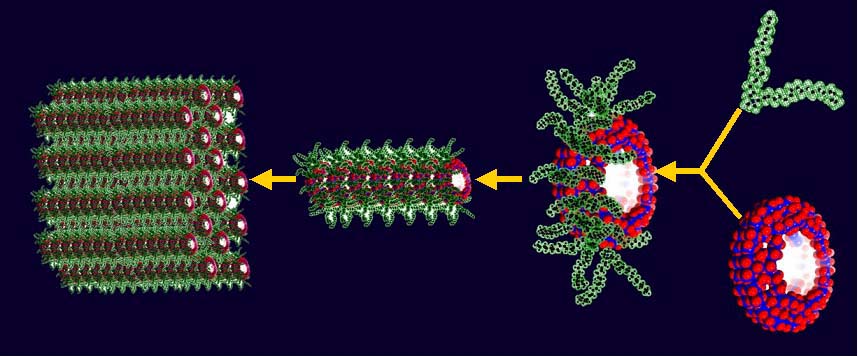
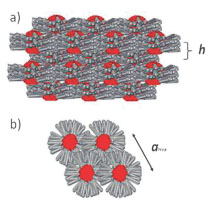
Liquid crystalline organization of clusters DODA-Mo132.
(a) View parallel to the layers illustrating the long range layered organization.
(b) View perpendicular to the layers highlighting the local hexagonal packing.
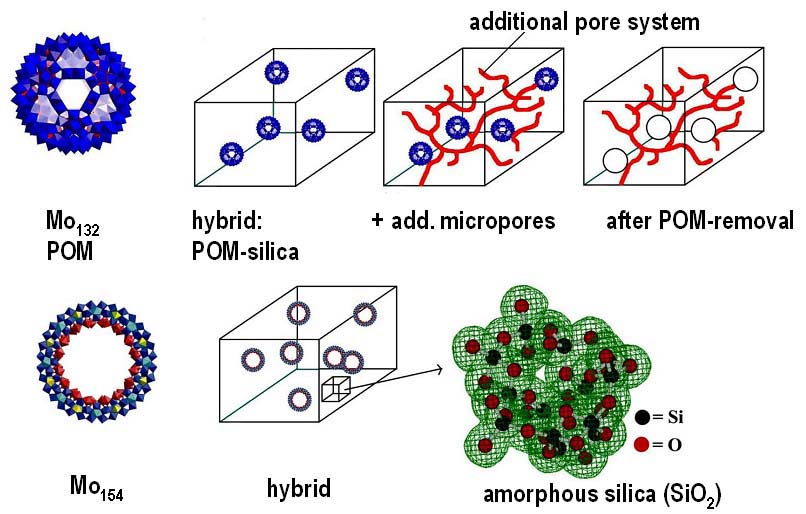
Construction of hybrid materials obtained by sol-gel processes, while the clusters' integrities as well as reactivities are maintained.
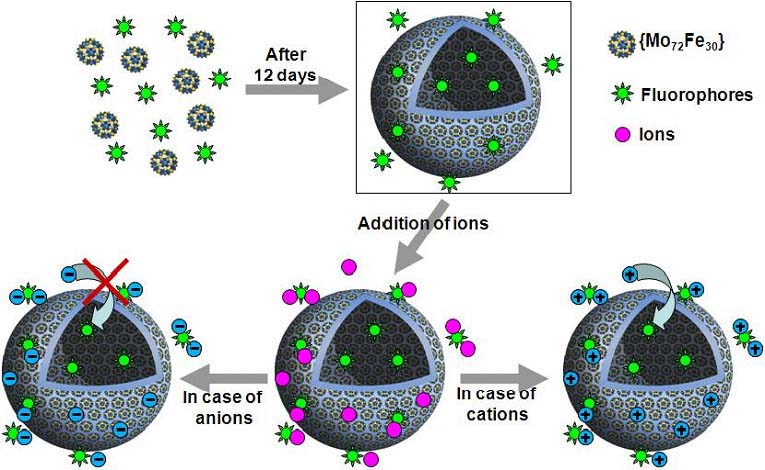
Study of cation transport through inorganic membranes/vesicles formed from {Mo72Fe30} Keplerates.
Important in the context: A theoretical study of the formation/stabilization mechanisms
of the mentioned new type of vesicles.
Publication:
Abstract: "We show that the equilibrium size of single-layer shells composed of polyoxometalate macroions is inversely proportional to the dielectric constant of the medium in which they are dispersed. [...] This observation points to a new class of thermodynamically stable shell-like objects. We point out the possible relevance our findings have for certain surfactant systems."
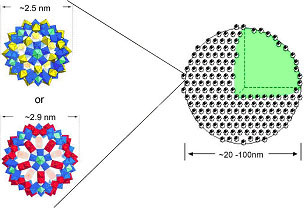 Fig. 1 Polyoxometalates Mo72Fe30 (top, left) and Mo132 (bottom, left) and a cartoon of their shell-like superstructure where the size range refers to the situation in aqueous solutions. |
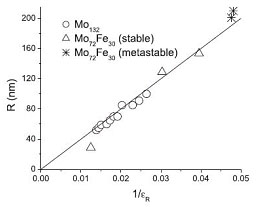 Fig. 2 Shell radius as determined by dynamic light scattering as a function of the inverse relative dielectric constant of the solvent (water, methanol, ethanol, propanol, acetone, acetone-water mixtures) for two different polyoxometalates: Mo72Fe30 and Mo132. |
Preparation of unprecedented supramolecular compounds.


Publication:
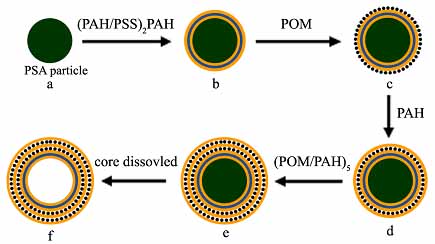 Scheme 1: Illustration of the LbL assembly of PAH, PSS and POM ({Mo72Fe30}) on PSA particles and hollow microcapsules. The first stage (a-b) involves stepwise deposition of PE on PSA colloidal particles. (b-e) Alternative assembly PAH and {Mo72Fe30} nanoparticles. (e-f) Decomposition of PSA cores and formation of hollow [(PAH/PSS)2(PAH/{Mo72Fe30})6PAH] microcapsules. |
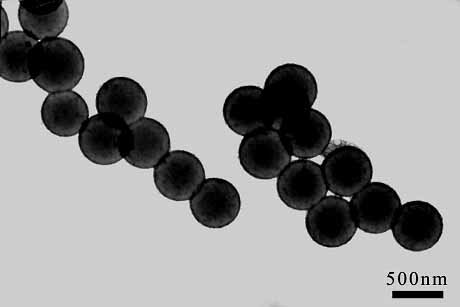 Fig. 6 TEM image of {Mo72Fe30}-embedded nanocapsules aligned in an external magnetic field. |
Modeling of water ligand exchange rates on mineral surfaces with relevance for geochemical aspects.
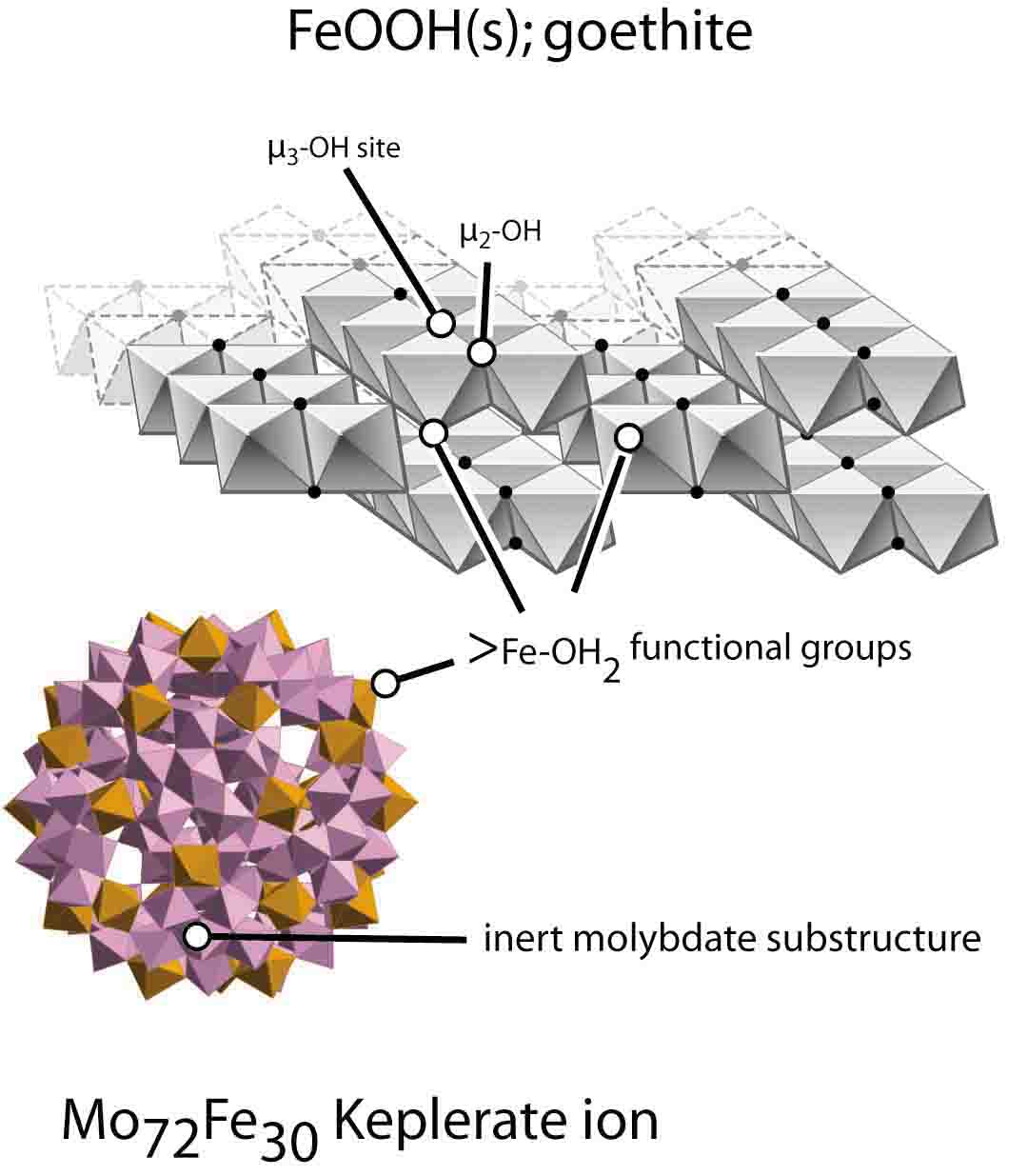
Figure 11 of the paper with the legend: Balogh et al. examined the rates of water exchange on a Keplerate molecule that had thirty >FeIII - OH2 accessible to the bulk aqueous solution. The molecule was intenden to shed light on the rates of ligand substitution in ferric hydroxide soil minerals, like the geothite structure shown, which also have isolated >FeIII - OH2.
See also: "Rates of Ligand Exchange between >FeIII - OH2 Functional
Groups on a Nanometer-Sized Aqueous Cluster and Bulk Solution"
E. Balogh, A. M. Todea, A. Müller, W. H. Casey (Inorg. Chem. 2007, 46, 7087).
The Graphical Abstract shows:
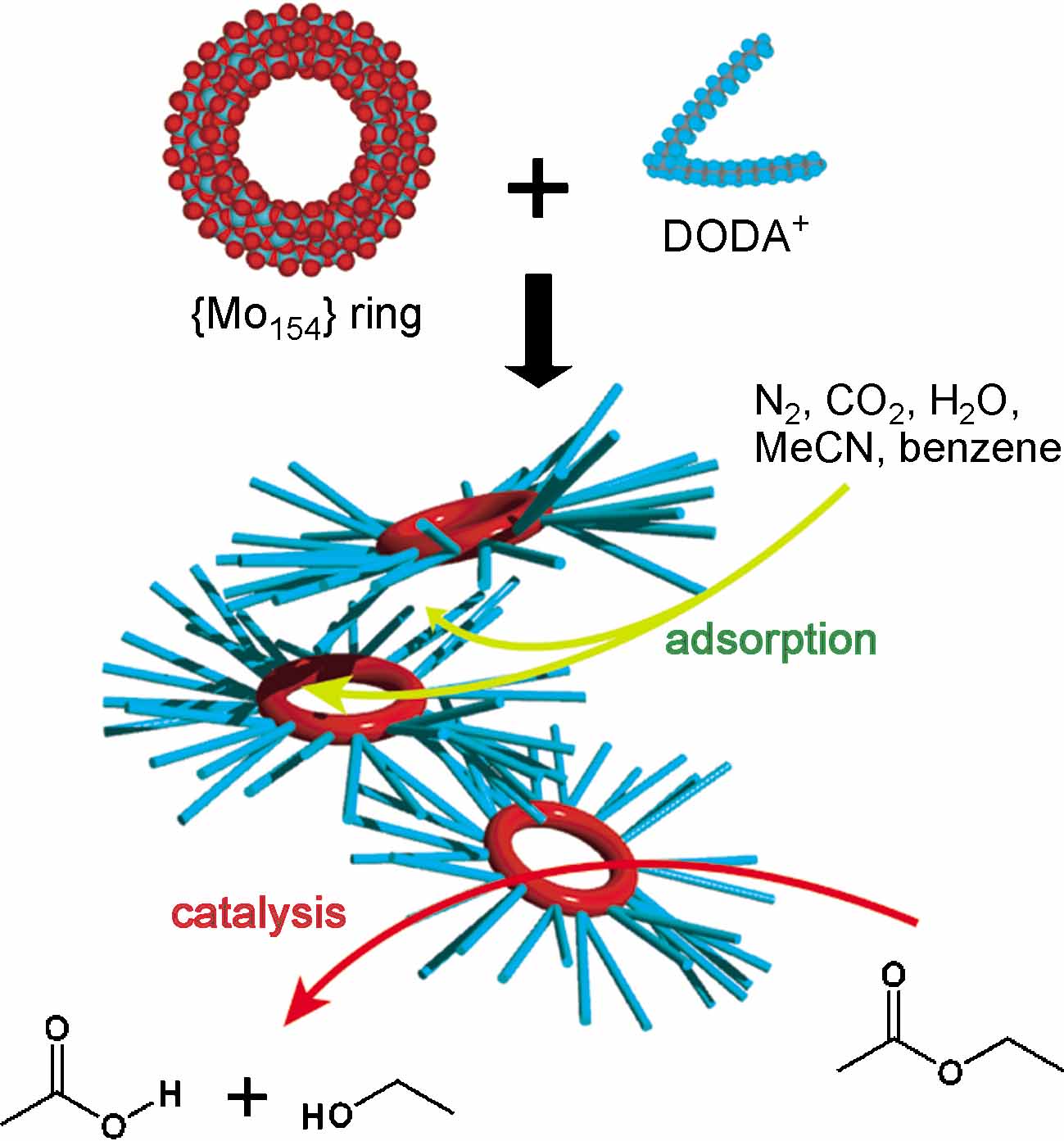 | Functional inner space: A gigantic ring-shaped {Mo154} polyoxometalate cluster anion can be stabilized by encapsulation in dimethyldioctadecylammonium (DODA) cations. Its inner nanospace enables adsorption of gases and vapors, and it acts as a water-tolerant solid acid catalyst . |
In context with our interest for the high formation tendency of spherical structures we wrote in the abstract of a related paper:
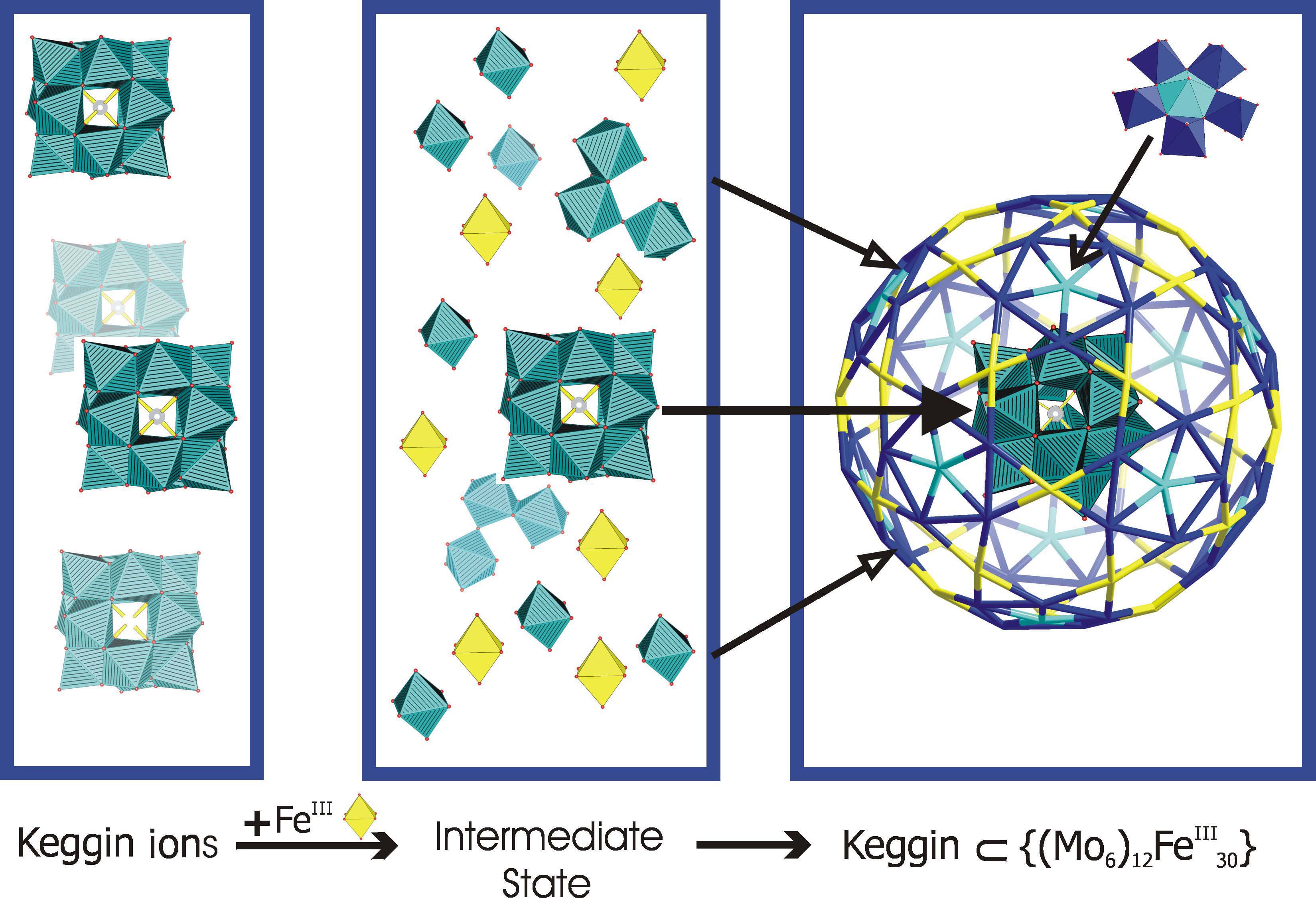
"Generation of cluster capsules (Ih) from decomposition products of a smaller cluster (Keggin-Td) while surviving ones get encapsulated: species with core-shell topology formed by a fundamental symmetry-driven reaction" (Chem. Commun. 2001, 657) the following:
Publication:
We read there: "Such a self-optimisation
(the formation of guanine quartet-based hydrogels)
behaviour may be of broader significance, namely for
prebiotic chemical evolution, whereby selection is driven
by phase cohesion, the entity selected being that giving the most
stable organised supramolecular assembly in a sort of prebiotic
Darwinism driven by self-organisation. The supramolecular
organisation drives the selection of the components giving the "
fittest" constituent."60 (Ref. 60: For systems
presenting a type of supramolecular Darwinism, see: A. Müller, S. K.
Das, H. Bögge, M. Schmidtmann, A. Botar, A. Patrut, Chem. Commun. 2001, 657).
In the following figure the process published in the mentioned Chem. Commun. paper is shown.
Regarding catalytic properties of Keplerates.
Regarding electronic structures of giant Keplerates.