Cyclopentadienyl Complexes of Divalent Silicon
The chemistry of low- and even zero-valent silicon (Si2+, Si1+, Si0) has seen great progress during
the last decades by the introduction of substituents and/or neutral ligands suitable for the thermodynamic and kinetic stabilization of
low molecular weight compounds.
In our group, π-complexation with cyclopentadienyl groups is the tool to stabilize neutral and ionic divalent
silicon compounds possessing sandwich- and half-sandwich structures. Important steps have been the synthesis of
the sandwich decamethyl-silicocene (Me5C5)2Si (1) and of the half-sandwich salt
(Me5C5)Si+ B(C6F5)4- (2) (Me5C5 = Cp*).
Chemistry of (Me5C5)2Si (1)
Compound 1 is the first monomeric silicon(II) species stable under ordinary conditions of temperature and pressure and at the same time also the first π-complex of silicon [1]. Two synthetic approaches are meanwhile available; one starting from a silicon(IV) compound [1], the other from carbene-stabilized silicon(II) species [2], as described in Scheme 1 (equations 1 and 2).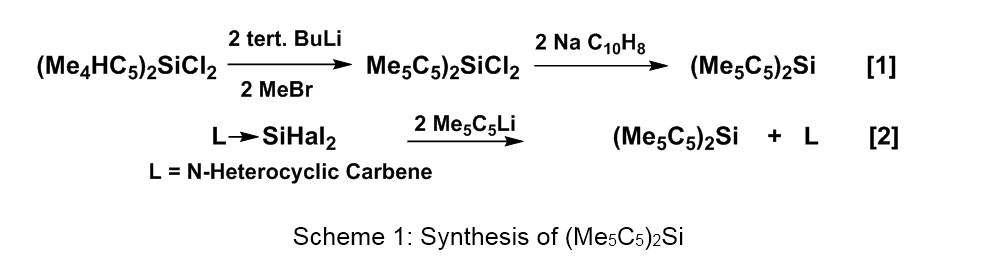
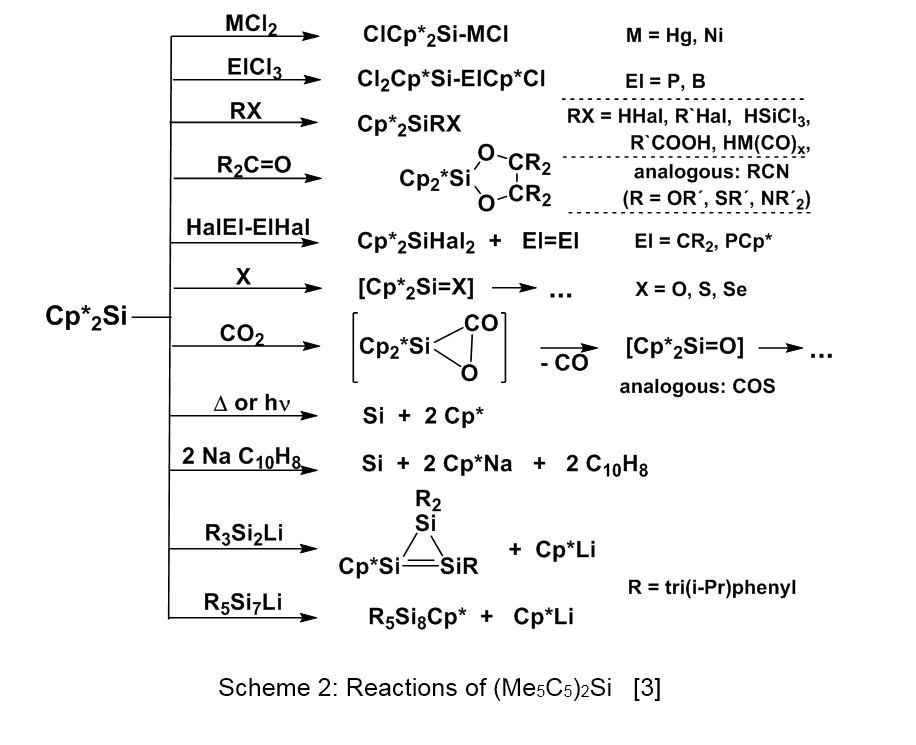
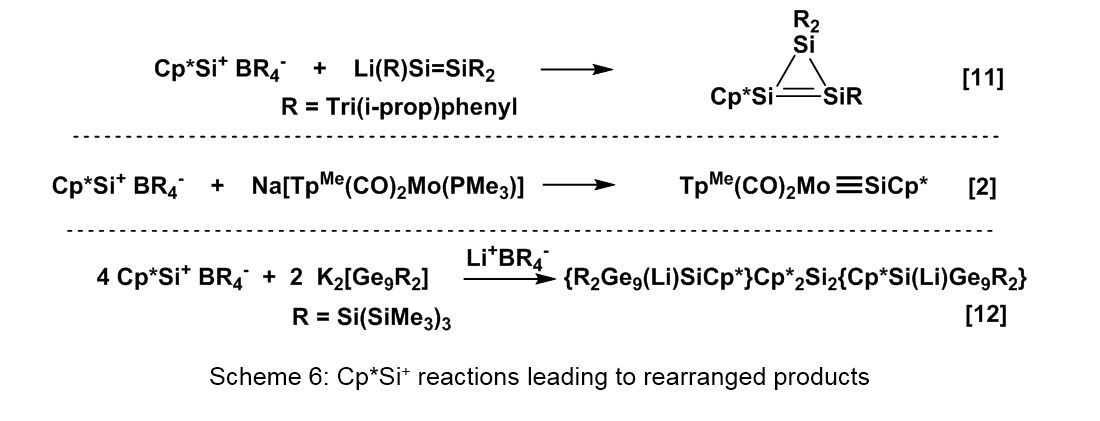
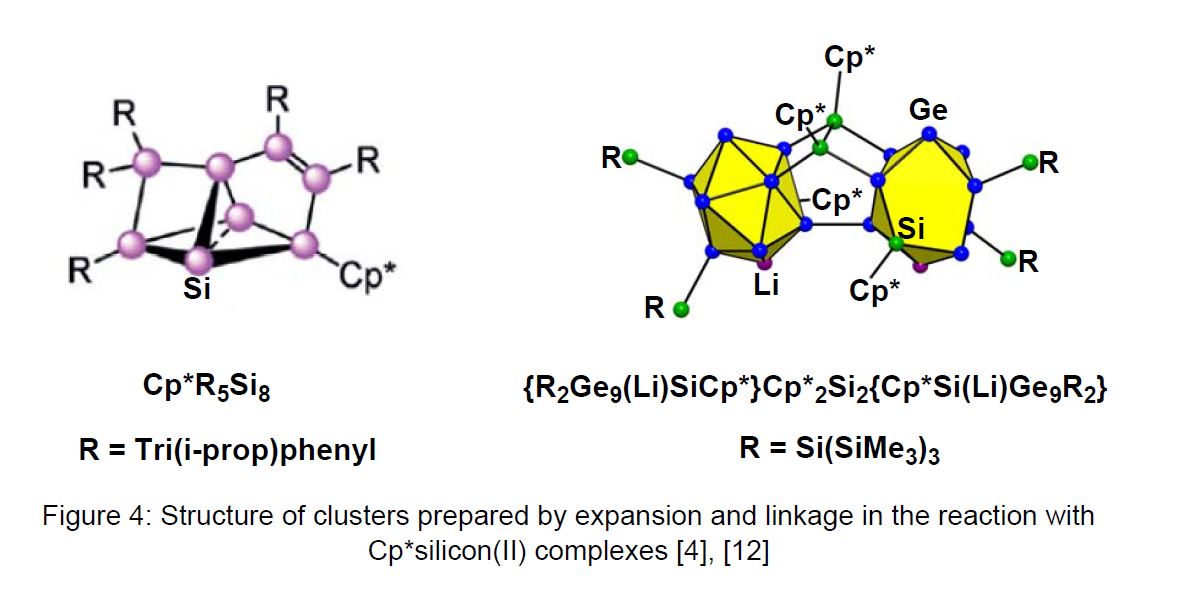
In most of the reactions, the electron-rich compound 1 behaves like a nucleophilic silylene with Cp* substituted silicon(IV) compounds as products (see scheme 2) [3]. Only in few cases, a Cp* elimination or substitution is observed, leading to elemental silicon or to low-valent silicon compounds (see scheme 1). Of special interest are the reactions of 1 with lithiated silicon-cluster compounds, which lead under Cp*Li elimination to neutral Cp*Si-insertion products, as observed in the group of Prof. D. Scheschkewitz (University of Saarbrücken) [4]. The structure of the Cluster R5Si8Cp* is presented in Figure 4. This strategy allows the stepwise and thus atomically precise expansion of silicon clusters.
Chemistry of (Me5C5)Si+ B(C6F5)4- (2)
The cationic half-sandwich complex Cp*Si+ can only be stabilized in the presence of anions with weak nucleophilicity; several approaches are described in Scheme 3.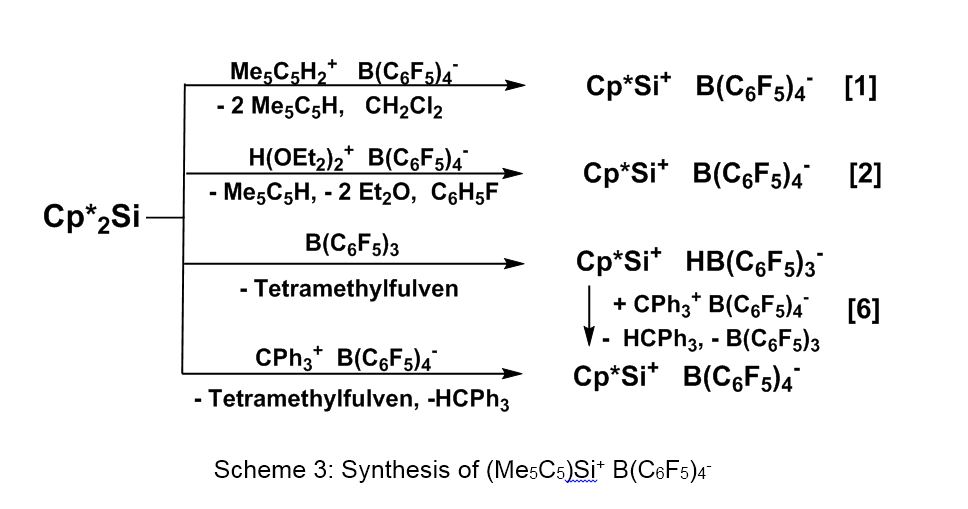
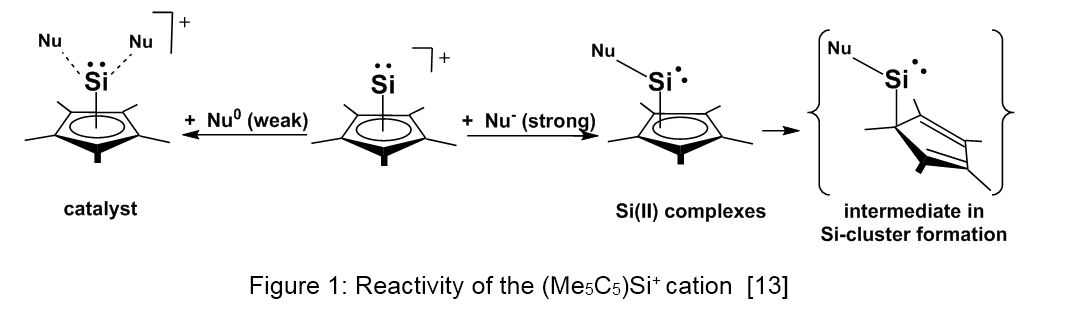
The Cp*Si+ cation behaves as a more or less strong electrophile - depending on the haptotropic changes within the Cp*Si unit - and offers an entrance into several fields of organosilicon chemistry (see Figure 1). In the reaction with rather weak neutral nucleophiles, Cp*Si+ behaves as a catalyst in novel transformations. In the reaction with anionic nucleophiles, stable or transient silicon(II) species are formed depending on the nucleophilicity and the steric bulk of the reagent.
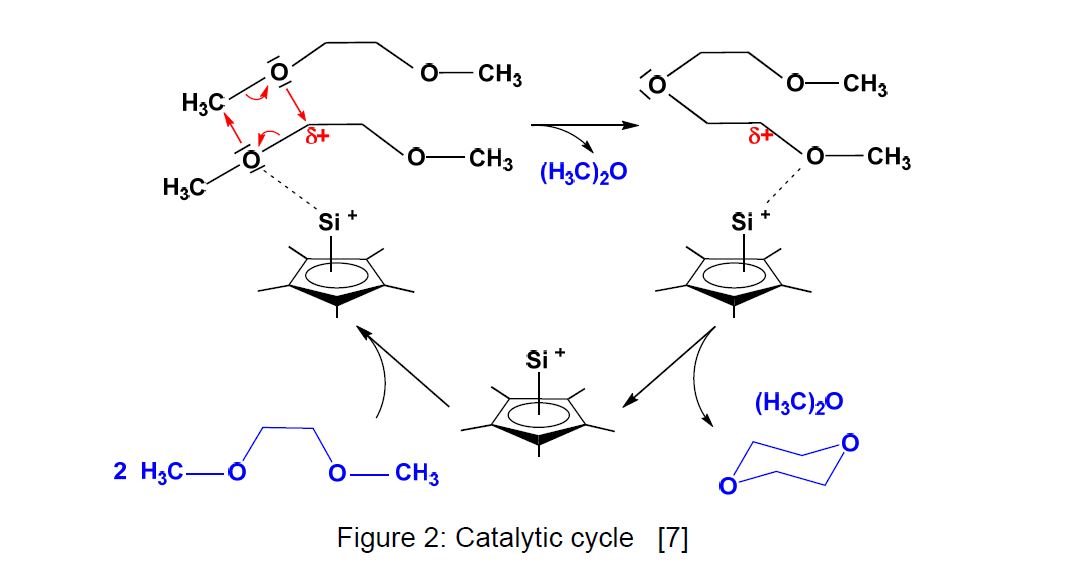
The catalytic function of Cp*Si+ was first observed in its behaviour against dimethoxyethane and several oligo(ethyleneglycol) diethers (Scheme 4). A weak interaction is regarded as the first step in the catalytic cycle, which is presented in Figure 2 [7].
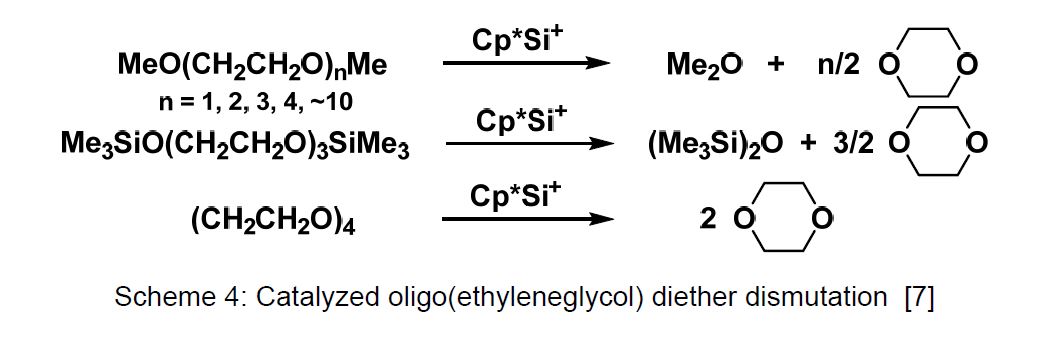
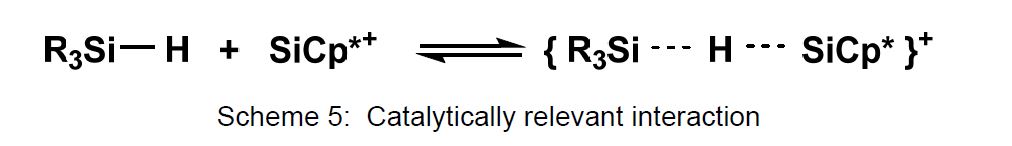
A weak interaction of the Cp*Si+ cation with the Si-H unit in organo(hydrido)silanes (Scheme 5) is the first step in the catalytic hydrosilylation of alkenes and in Piers-Rubinsztajn reactions. A detailed study concerning the application of these reactions in silicone chemistry is presented from the research group of Dr. E. Fritz-Langhals, Wacker Chemie AG [6].
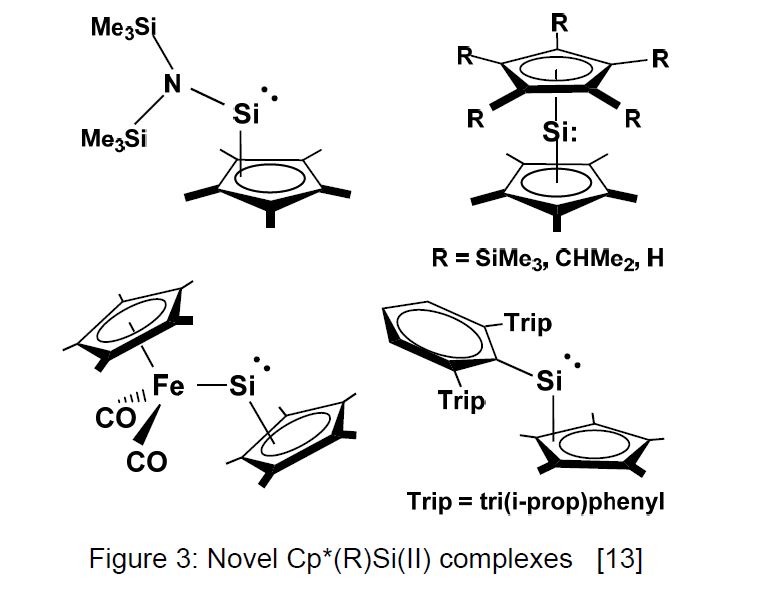
Novel classes of silicon(II) complexes prepared from Cp*Si+ are collected in Figure 3. The aminosilicon(II) complex is a monomeric species in solution and in the gas phase, but dimerizes in the solid state under Si=Si bond formation [8]. The ferriosilicon(II) complex shows a small HOMO-LUMO gap with the result of a low-field 29Si NMR shift [9]. The mixed sandwich compounds Cp*Si CpR should allow the preparation of other than Cp* substituted cationic species, as proven by the isolation of a salt containing the (iProp)5C5Si+ cation [10].
Transient Cp*(Nu)Si species (see Scheme 3) are postulated in reactions, where rearrangement processes determine the final structure (see Scheme 6, Figure 4). The reaction of 2 with the lithium disilenide Li(R)Si=SiR2 leads to a Cp*-substituted cyclotrisilene, which can be transferred to silicon cluster compounds using Cp* as leaving group [11]. Reaction of 2 with a specially designed transition-metal complex leads to a species containing a triple-bond unit M≡SiCp* [2]. Reaction of 2 with the Zintl-type cluster
The search for the Cp*silicon(I) complex Cp*2Si2 (3)
The synthesis of the silicon(I) complex Cp*2Si2 was unsuccessful so far (see Scheme 7) and remains an interesting target [13]. A controlled disproportionation of a donor-stabilized complex might lead to the formation of self-assembled, atom-precise silicon clusters.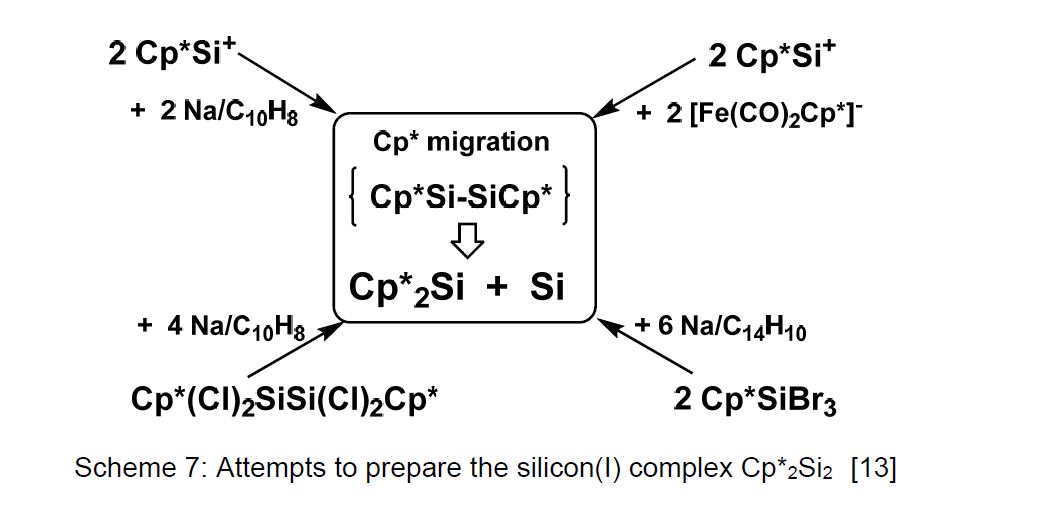
Summary
π-Complexation with cyclopentadienyl ligands is a useful tool to stabilize neutral and cationic silicon(II) species. By this strategy, novel classes of compounds have become available, and novel reaction principles have been observed. Chemistry, structure and bonding of 1 is summarized in [3], that of 2 in [13]. A further successful development of this tool is expected.Literature:
[1] P. Jutzi, D. Kanne, C. Krüger, Angew. Chem. 1986, 98, 163.[2] A.C. Filippou et al., Organometallics 2018, 37, 772.
[3] T. Kühler, P. Jutzi, Adv. Organomet. Chem. 2003, 49, 1.
[4] K. Leszczynska et al., Angew. Chem. Int. Ed. 2019, 58, 5124.
[5] P. Jutzi, A. Mix, B. Rummel, W.W. Schoeller, B. Neumann, H.G. Stammler, Science 2004, 305, 849.
[6a] E. Fritz-Langhals, Org. Process Res. Dev. 2019, 23, 2369, DOI:10.1021/acs.oprd.9b00260
[6b] E. Fritz-Langhals, Org. Process Res. Dev. 2020, 24, 1484, DOI:10.1021/acs.oprd.0c00214
[7] K. Leszczynska, A. Mix, R.J.F. Berger, B. Rummel, B. Neumann, H.-G. Stammler, P. Jutzi, Angew. Chem. Int. Ed. 2011, 50, 6843.
[8] P. Jutzi, A. Mix, B. Neumann, B. Rummel, W.W: Schoeller, H.G. Stammler, A.B. Rozhenko, J. Amer. Chem. Soc. 2009, 131, 12137
[9] P. Jutzi, K. Leszczynska, A. Mix, B. Neumann, B. Rummel, W. Schoeller, H.-G. Stammler, Organometallics 2010, 29, 4759.
[10] P. Jutzi, A. Mix, B. Neumann, B. Rummel, H.-G. Stammler, Chem. Commun. 2006, 3519.
[11] K. Leszczynska, K. Abersfelder, A. Mix, B. Neumann, H.-G. Stammler, M.J. Cowley, P. Jutzi, D. Scheschkewitz, Angew. Chem. Int. Ed. 2012, 51, 678.
[12] S. Frischhut, P. Jutzi, T.F. Fässler, 9. European Silicon Days, 2018, Saarbrücken, Poster P12.
[13] P. Jutzi, Chem. Eur. J., 2014, 20, 9192.
