Metal and Metal-Oxide Nanoparticles Prepared from Solution by Precursor Decomposition; and: the "Droplet Model"
Control of the diffusion process in Co nanoparticles and of the redox-transmetalation process in the preparation Co@Ru core-shell particles
For the preparation of cobalt-ruthenium core-shell particles synthesized by the redox-trans-metalation process [1],
a high crystallinity of the core component and the kinetic control of the redox-reaction on the core-surface are
prerequisites to avoid the unwanted diffusion of the shell component and the formation of a CoRu alloy [2].
To improve the crystallinity of the cobalt core, some parameters in the thermal decomposition of dicobaltoctacarbonyl
and of some other Co substrates in organic solvents are improved (control by HRTEM). Furthermore, the transmetalation
process on the Co surface (Co0 + Ru2/3+ → Co2+ + Ru0) with
several Ru2/3+ sources is studied in more detail.
[1] W. Lee, M.G. Kim, J. Choi, J.-Il Park, S.J. Ko, S.J. Oh, J. Cheon, J. Am. Chem. Soc. 2005, 127, 1609.
[2] D. Zitoun, C. Amiens, B. Chaudret, M.C. Fromen, P. Lecante, M.J. Casanove, M. Respaud, J. Phys. Chem. 2003, 107, 6997.
Synthesis of multidentade ligands for the kinetic stabilisation on the surface of hard and soft nanoparticles
In recent years much attention has been paid to the synthesis, characterization, and application of metal and metal
oxide nanoparticles. Amphiphilic long-chain organic molecules play an important role as ligands of nanoparticles to
prevent their agglomeration and to provide the necessary functionalization. Long-chain organothiols are suitable ligands
for "soft" metal surfaces and long-chain carboxylic acids for "hard" metal and metal oxide surfaces.
With respect to possible applications, there is further demand for sufficient surface coverage of the particles, for strong
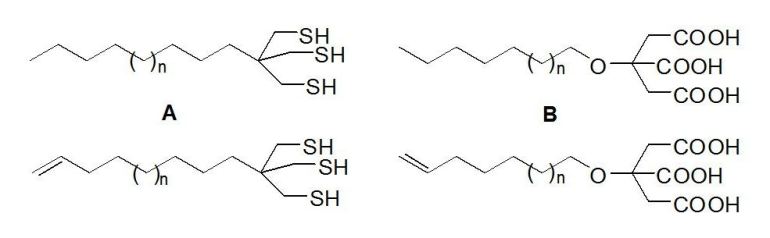
particle-ligand bonding, and for low exchange rates. In this context we have synthesized alkenyl-functionalized and
non-functionalized long-chain tridentade organotrithiols A [1] and citric acid ethers B [2].
These ligands show an excellent thermodynamic and kinetic stability. The terminal alkene groups enable further functionalization.
[1] K. Wojczykowski, P. Jutzi, Synlett 2006, 39.
[2] D. Meißner, P. Jutzi, Z. Naturforsch. 2009, 64 b, 731.
Size and shape control in the synthesis of β-tin particles prepared by precursor-decomposition in solution via nanoscaled droplets
Nucleation is the very first stage in any crystallization process. In nanoparticle synthesis by precipitation procedures
in solution, the control of the nucleation and of the growth process is necessary to get monodisperse particles with a
well-defined and reproducible morphology. To understand the underlying processes, LaMer and Dinegar [1] applied classical
nucleation theory to qualitatively describe the kinetics of burst nucleation and diffusional growth. The drawn conclusions
are based upon an extensive study of the synthesis of monodispersed sulphur colloids. In the now generally
accepted "LaMer model", crystalline nuclei are important constituents in the supersaturated nucleation regime.
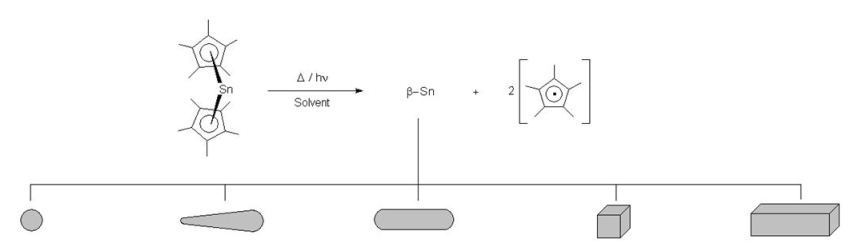
We have prepared nano- and microcrystalline tin particles with different morphology by thermal or photochemical
decomposition of decamethylstannocene in organic solvents:
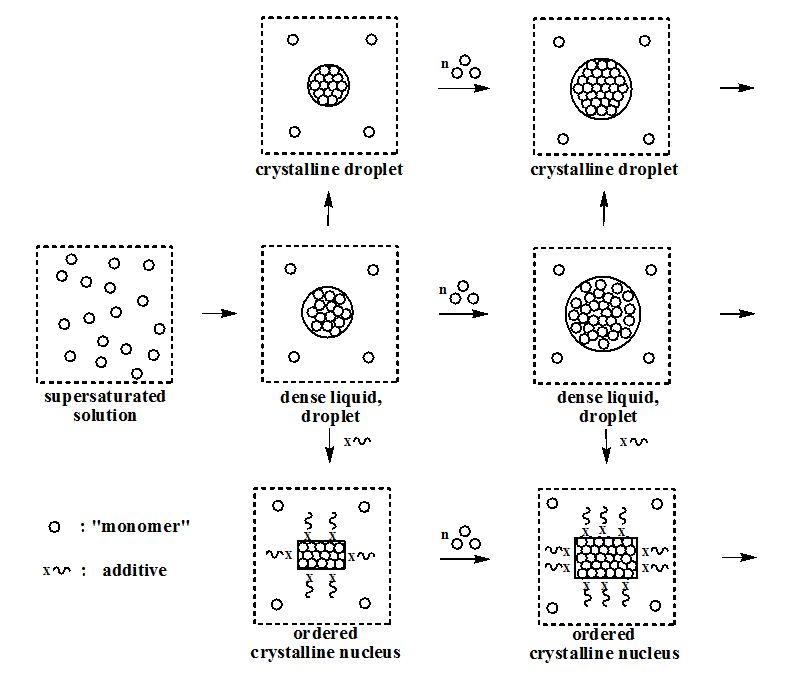
Our experiments demonstrate, that - in contrast to expectations based on the LaMer model - the initial structure of a
tin-nucleus is liquid-like. The further interaction of droplets with "monomers" or with other droplets or with additives
determines the final morphology. Size and shape of the resulting crystalline material (spheroids, cubes, rectangular bars,
round bars, needles (conic round bars) can be controlled by the reaction parameters (solvent, concentration, temperature,
additives, ). In later stages of the process, the droplet-like particle becomes entirely crystalline [2].
Thus, nanostructured tin-droplets play an important role in the very first stage of particle formation [3], in the growth
process and also in the final structure formation.
The synthesis of monodispersed spherical colloids of low-melting metals like Pb, In, Sn, and Cd in the diameter range
of 100-600 nm is already described in literature [4]. Hints for the presence of "fluid droplets" during the nucleation
stage stem from theoretical simulations and from experiments with colloidal polymer suspensions [3,5,6,7].
To the best of our knowledge, the role of droplets concerning the shape control in particle formation is described
here for the first time.
Having in mind the well-known melting point depression in nano-particles [8,9] and the super-cooling effect
observed for several elemental metallic liquids [10], the presence of liquid-like nuclei during the initial
stage of particle formation in solution seems to be a general phenomenon even for such metals and metal compounds which
melt in the bulk at higher temperatures (> 1000 °C). It has to be checked experimentally, whether the "droplet phase"
can be used to determine the final morphology of such nano-particles, in addition to the so far known strategies [11].
The following scheme presents our modified mechanistic view of particle nucleation:
[1] V.K. LaMer, R.J. Dinegar, J. Am. Chem. Soc. 1950, 72, 4847.
[2] A. Dreyer, A. Hütten, P. Jutzi, in preparation.
[3] "Nucleation: What happens at the Initial Stage?", T.H. Zhang and X.Y. Liu, Angew. Chem. 2009, 121, 1334.
[4] Y. Wang, Y. Xia, Nano Letters 2004, 4, 2047.
[5] P.R. ten Wolde, D. Frenkel, Science 1977, 277, 1975.
[6] U. Gasser, E.R. Weeks, A. Schoflied, P.N. Pusey, D.A. Weitz, Science 2001, 292, 258.
[7] V.J. Anderson, H.N.W. Lekkerker, Nature 2002, 416, 811.
[8] For example: Melting point of bulk Au 1064 C; mp of Au nanoparticles ∼360 C [9] .
[9] Ph. Buffat, J.P. Borel, Phys. Rev. A, 1976, 13, 2287.
[10] R. Kalyanaraman, J. Appl. Phys. 2008, 104 , 033506.
[11] Y. Xia, Y. Xiong, B. Lim, S.E. Skrabalak, Angew. Chem. Int. Ed. 2009, 48, 60.
