Dynamic Covalent Chemistry: A Strategy for the Synthesis of Novel Organogallium Compounds
"Dynamic Covalent Chemistry" relates to chemical reactions carried out reversibly under conditions of equilibrium
control. The reversible nature of chemical reactions introduces the prospects of "error checking" and "proof reading"
into synthetic processes where dynamic covalent chemistry operates. Since the formation of products occurs under
thermodynamic control, product distributions depend only on the relative stabilities of the final products [1]. To the
best of our knowledge, this very successful concept has so far not been applied in the field of Organometallic
Chemistry, i.e. for the synthesis of structurally interesting compounds containing metal-carbon bonds.
A prerequisite for the application of the concept of "Dynamic Covalent Chemistry" are at least two reactive groups at
the reactive centers involved in the dynamic process. Furthermore, some steric constraints at the reaction
centers (fixed geometry) are indicated for an easier control of product formation.
We have selected two classes of compounds from organogallium chemistry to check the above described
concept: multiple dialkylgallyl substituted benzenes and twofold dialkylgallyl substituted ferrocenes.
Dialkylgallyl Substituted Benzenes and their Condensation Products
The following bis- and tris(dialkylgallyl) substituted benzene derivatives are investigated:
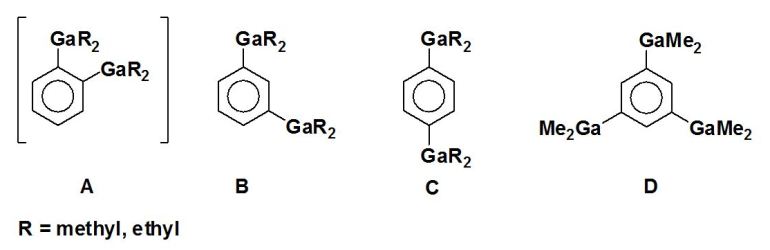
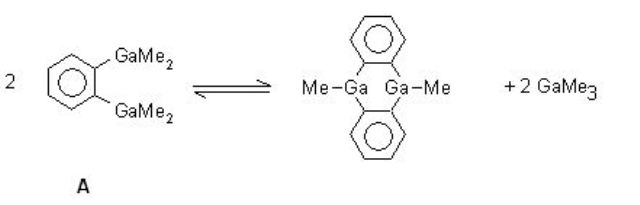
In compounds of type A, condensation products of the 9,10-dihydro-9,10-R2-9,10-digalla-anthracene type
are formed under normal conditions of temperature and pressure [2].
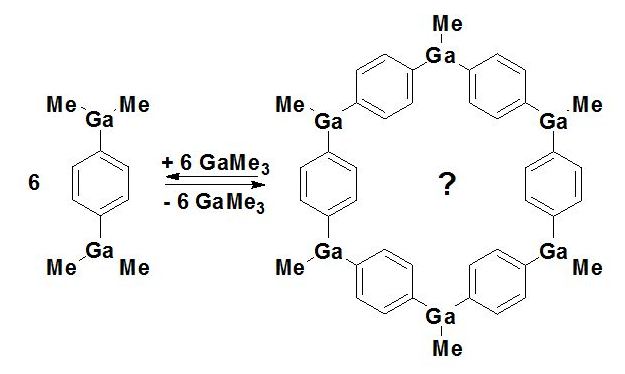
In compounds of type B and C, the equilibrium is shifted to polymeric condensation products of undefined
structure. Addition of excess trialkylgallium (the trialkylgallium compound as solvent !) to the polymers allows
the isolation of B, C and D as rather thermolabile and very air-sensitive crystalline compounds,
which easily undergo condensation reactions [3,4]. The experimental conditions for a controlled condensation
of B, C and D to oligomers with a defined ring structure are under investigation:
A network structure is expected in case of compound D.
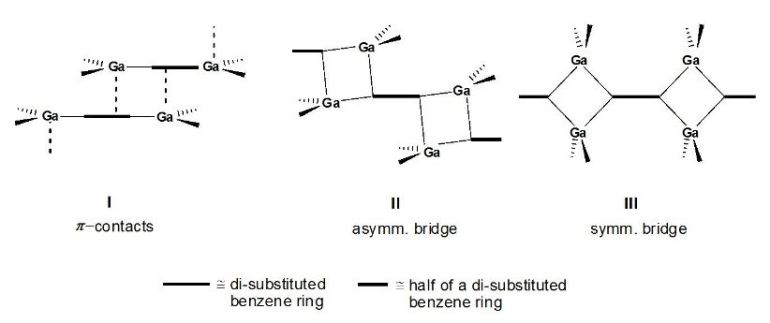
It is interesting to note, that different structural features have been observed in the solid-state structures
of comparable dimethylgallyl- and diethylgallyl-substituted benzenes of type B and C [3,4] and also for
the condensation products of type A [2]: Methyl or ethyl as substituent at gallium makes the difference!
A schematic presentation is given in Figure 1.
Figure 1
Dialkylgallyl Substituted Ferrocenes and their Condensation Products
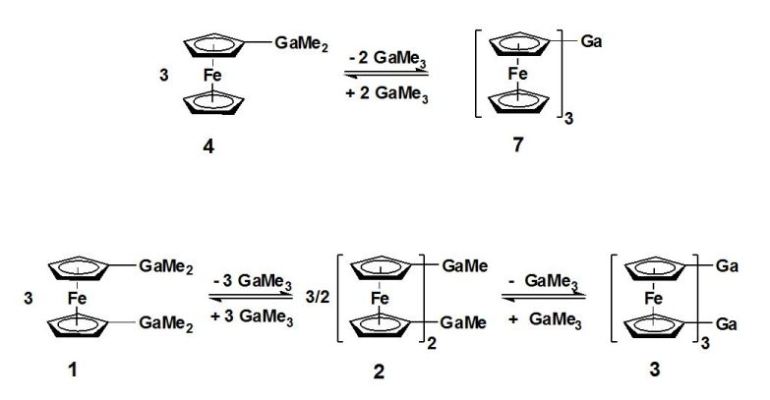
Starting from the isolable compounds dimethylgallylferrocene (4) and 1,1-bis(dimethylgallyl)-ferrocene (1),
the reversible reactions to the twofold (2) and threefold (7, 3) ferrocen(edi)yl substituted gallium
compounds have been investigated [5].
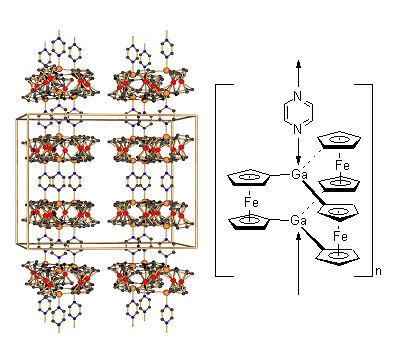
The ferrocenophane 3 reacts with monofunctional bases like pyridine to carousel-type complexes and with difunctional
Lewis bases like pyrimidine to supramolecular adducts (see Figure )[5].
[1] S.J. Rowan et al., Angew. Chem. Int. Ed. 2002, 101, 898.
[2] P. Jutzi, H. Sielemann, B. Neumann, H.G. Stammler, Inorg. Chem. Acta 2005, 358, 4208.
[3] P. Jutzi, J. Izundu, H. Sielemann, B. Neumann, H.G. Stammler, Organometallics 2009, 28, 1985.
[4] J. Izundu, P. Jutzi, B. Neumann, H. Sielemann, H.G. Stammler, Z. Naturforsch. B 2009, 64b, 1375.
[5] A. Althoff, D. Eisner, P. Jutzi, N. Lenze, B. Neumann, W.W. Schoeller, H.G. Stammler, Chem. Eur. J. 2006, 12, 5471.
